Figure 1.
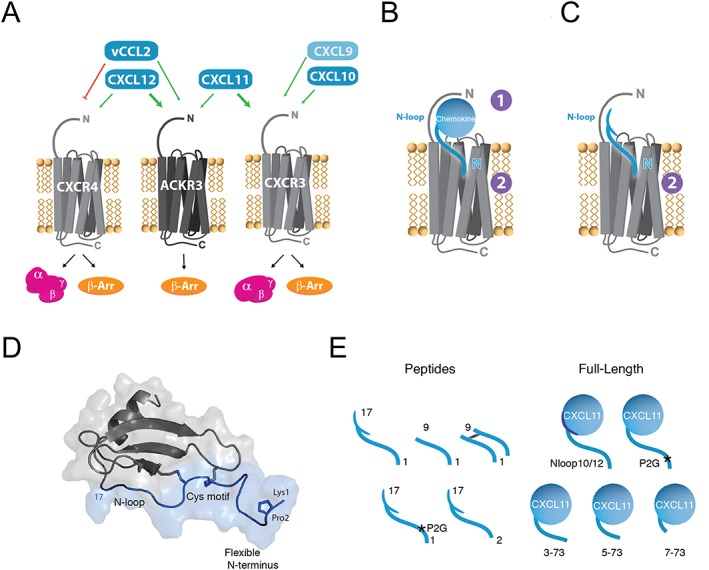
CXCR4‐ACKR3‐CXCR3 receptor‐ligand interaction network and chemokine N‐terminal features. (A) Selectivity and crosstalk between the two canonical G protein‐signalling chemokine receptors, CXCR4 and CXCR3, the atypical β‐arrestin‐biased receptor ACKR3 and their shared ligands. CXCL12 is the only endogenous chemokine ligand for CXCR4. It is also the highest affinity chemokine for ACKR3 but does not bind CXCR3. The viral chemokine vCCL2 is a CXCR4 antagonist but acts as an agonist of ACKR3. CXCL11 is the dominant ligand for CXCR3 and binds also to ACKR3. CXCL10 and CXCL9 bind and activate CXCR3, but not ACKR3. (B) Two‐site/two‐step model for the interactions of full‐length chemokines with their cognate receptors. In the first step, the body of the chemokine and the N‐loop are specifically recognized by the N terminus of the receptor (CRS1). During the second step, the insertion of the chemokine N terminus into the receptor‐binding pocket (CRS2) stabilizes its active form triggering downstream signalling. (C) Peptides derived from the N‐terminal region of chemokines, which represent useful probes to investigate the interaction between chemokines and receptors. (D) Schematic representation of CXCL12 and location of the N‐terminal features investigated in this study (blue). The N‐terminal region encompasses the flexible N terminus (1–8), the CXC cysteine motif (9–11) and the N‐loop (13–17). (E) Chemokine‐derived peptides and CXCL11 variants investigated in this study.
