Figure 4.
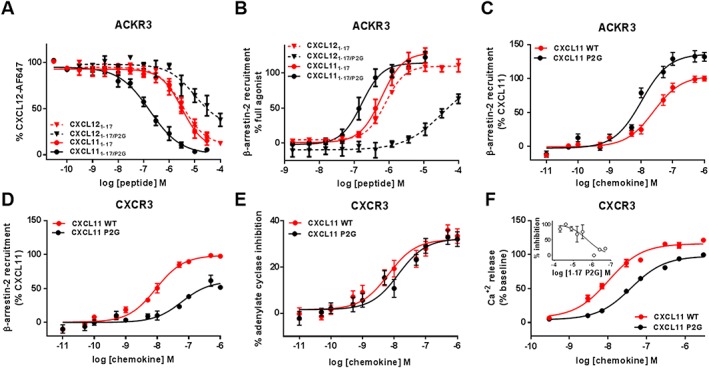
Binding and activation of ACKR3 and CXCR3 by N‐terminal peptides and full‐length CXCL11 bearing the P2G mutation. (A and B) Comparison of the effects of the P2G mutation in CXCL12‐ and CXCL11‐derived peptides on (A) binding to ACKR3 assessed by competition studies with Alexa Fluor 647‐coupled CXCL12 and (B) β‐arrestin‐2 recruitment to ACKR3 monitored by NanoLuc complementation in U87 cells. (C and D) Comparison of β‐arrestin‐2 recruitment to ACKR3 (C) and CXCR3 (D) induced by CXCL11WT and P2G mutant in HEK293 cells monitored by BRET using receptor‐YFP fusion constructs and β‐arrestin‐2‐Rluc. (E and F) CXCR3 activation by CXCL11WT and P2G mutant monitored in HEK293 cells through (E) adenylate cyclase inhibition using BRET reporter GFP10‐Epac‐ Rluc3 and (F) intracellular calcium release using FLIPR 4 Calcium Flux kit dye. (F‐inset) Antagonist properties of peptide CXCL111–17/P2G monitored in calcium assay. Each experiment was performed five times, and the data shown are means ± SEM.
