Figure 7.
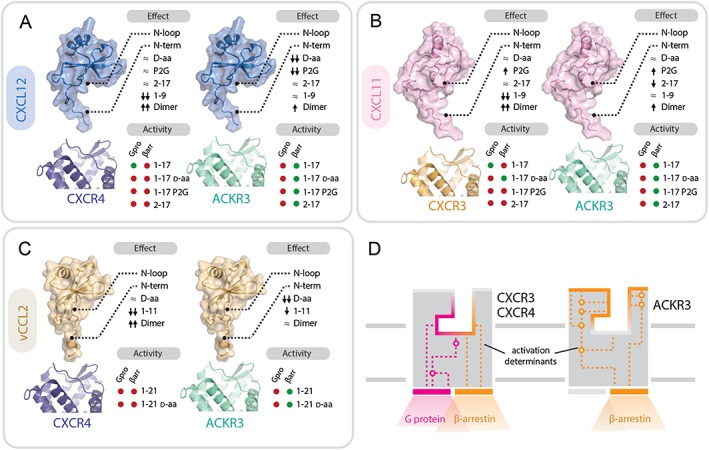
Contributions of chemokine and receptor regions to binding and activation of ACKR3, CXCR3 and CXCR4. (A–C) Importance of structural determinants of chemokines CXCL12 (A), CXCL11 (B) and vCCL2 (C) for binding and activity towards the receptors. Upper diagram: effects of the different modifications of chemokine N‐terminal domains on receptor binding and/or activity: ≈ no effect or <5‐fold change; ↑ or ↓ increase or decrease by >5 fold; ↑↑ or ↓↓ increase or decrease by >50 fold. Lower diagram: activity (shown as green circles) and lack of activity (shown as red circles) in G protein signalling (Gpro) and β‐arrestin recruitment (βarr) of peptides comprising the flexible N terminus and the N‐loop (1–17/21), their D stereoisomers and proximally modified variants (P2G, 2–17). (D) Comparison of ACKR3 and CXCR3/4 binding pockets. The activation determinants of ACKR3 are localized closer to the surface compared with CXCR3 and CXCR4. Ligand binding to ACKR3 pocket triggers a limited signalling repertoire (i.e. β‐arrestin‐2 recruitment). In contrast, CXCR4 and CXCR3 activation determinants are localized deeper in the ligand binding pocket with chemokine triggering G protein signalling and, subsequently, arrestin recruitment.
