Abstract
Background
The FLAME study compared once-daily indacaterol/glycopyrronium (IND/GLY) 110/50 μg with twice-daily salmeterol/fluticasone (SFC) 50/500 μg in symptomatic patients with moderate to very severe COPD and a history of exacerbations in the previous year.
Methods
This prespecified and post hoc subgroup analysis evaluated treatment efficacy on 1) moderate/severe exacerbations according to prior exacerbation history and treatment, and 2) types of exacerbations according to health care resource utilization (HCRU) during 1-year follow-up.
Results
IND/GLY reduced the rate of moderate/severe exacerbations versus SFC in patients with a history of 1 exacerbation (rate ratio [RR]: 0.83, 95% CI: 0.75–0.93), ≥2 exacerbations (RR: 0.85, 95% CI: 0.70–1.03) and ≥2 exacerbations or ≥1 hospitalization in the previous year (RR: 0.86, 95% CI: 0.74–1.00). Prolonged time-to-first exacerbation was observed in all the groups according to exacerbation history. Moderate/severe exacerbations decreased with IND/GLY versus SFC, independent of previous treatment. IND/GLY significantly reduced rates of moderate/severe exacerbations treated with antibiotics (RR: 0.79, 95% CI: 0.67–0.93) and systemic corticosteroids and antibiotics (RR: 0.80, 95% CI: 0.70–0.91); rates of exacerbations treated with systemic corticosteroids alone were comparable (RR: 0.99, 95% CI: 0.80–1.22).
Conclusion
Overall, IND/GLY demonstrated consistent beneficial effects versus SFC on moderate/severe exacerbations, independent of prior exacerbation history or treatment. The efficacy of IND/GLY on exacerbation prevention was superior to SFC for exacerbations treated with antibiotics with/without systemic corticosteroids and was similar for exacerbations treated with systemic corticosteroids alone.
Keywords: indacaterol/glycopyrronium, salmeterol/fluticasone, LABA/LAMA, LABA/ICS
Introduction
COPD is a complex heterogeneous disease, with a global prevalence of 10.7%.1 COPD exacerbations are acute events characterized by a worsening of respiratory symptoms – particularly dyspnea, cough and sputum production – that is beyond day-to-day variations and requires changes in medication.2 COPD exacerbations are thought to contribute to the progression of COPD and are associated with an increased risk of future exacerbations and hospitalizations, reduced quality of life, and higher morbidity and mortality. COPD exacerbations impose a high economic burden on health care systems.3
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017 strategy proposes that symptoms and exacerbation risk (estimated through exacerbation history including prior hospitalization) need to be assessed to determine disease severity and appropriate treatment.2 Reducing the risk of future exacerbations is one of the major goals in the management of this chronic disease. Due to the heterogeneous nature of COPD, treatment and management of exacerbations are dependent on a number of factors.4 The severity, frequency, duration, and likelihood of airway bacterial infection and type of underlying inflammation are/may be crucial factors that contribute to the heterogeneity of exacerbations. This heterogeneity also influences the treatment offered by physicians. Health care resource utilization (HCRU) during the exacerbation may depend on the maintenance treatment selected for exacerbation prevention. In the INSPIRE study, exacerbation rates (as defined by HCRU) were similar between the treatment groups (salmeterol/fluticasone [SFC] and tiotropium); however, the rate of exacerbations requiring treatment with antibiotics or systemic corticosteroids differed between the two groups.5
Long-acting bronchodilators, mainly long-acting β2 agonists (LABAs) and/or long-acting muscarinic antagonists (LAMAs), are the current standard of care for patients with COPD. The GOLD 2017 strategy recommends a combination of long-acting bronchodilators as a preferred first choice of treatment for more symptomatic patients with a high risk of exacerbation (GOLD Group D) and for patients not sufficiently controlled by monotherapy (GOLD Groups B and C).2
FLAME was a large Phase III study that demonstrated the superiority of a dual bronchodilator regimen, LABA plus LAMA (indacaterol/glycopyrronium [IND/GLY]; 110/50 μg once daily), over a LABA plus inhaled corticosteroid (LABA/ICS; SFC 50/500 μg twice daily) combination in preventing exacerbations in symptomatic (modified Medical Research Council [mMRC] dyspnea grade ≥2) COPD patients with an exacerbation history.6 The FLAME study reported an overall 17% reduction in the rate of moderate-to-severe exacerbations.6 In a secondary analysis, IND/GLY provided superior or similar benefits over SFC, independent of blood eosinophil levels.7 FLAME provides an opportunity to perform an in-depth analysis of the heterogeneity of exacerbations and the efficacy of the 2 compared treatments. In this subgroup analysis, we evaluated the efficacy of the 2 treatments on 1) moderate or severe exacerbations according to patients’ exacerbation history in the previous year and treatment prescribed prior to recruitment and 2) different types of exacerbations according to HCRU during the 1-year follow-up.
Methods
Study details
Details of the FLAME study design have been reported previously.6 Briefly, FLAME (NCT01782326) was a large Phase III, 52-week, multicenter, randomized, double-blind, double-dummy, parallel-group study. Following the 1-week screening and 4-week run-in periods, patients were randomized to receive either once-daily IND/GLY 110/50 μg or twice-daily SFC 50/500 μg for 52 weeks, with an additional 30-day follow-up period. The trial was approved by the ethics committee at each trial center (Table S1) and was conducted according to the ethical principles of the Declaration of Helsinki. All patients provided written informed consent. Prespecified and post hoc statistical analyses are outlined in Table 1.
Table 1.
Prespecified and post hoc statistical analyses
| Endpoint | Statistical analysis | Prespecified analysis | Post hoc analysis |
|---|---|---|---|
| Baseline demographic and clinical characteristics | Summaries by exacerbation history in the previous year | X | |
| Rate of moderate/severe exacerbations | Subgroup analysis by exacerbation history in the previous year • 1 and ≥2 moderate/severe exacerbations |
X | |
| Rate of all (mild, moderate, severe) exacerbations | • GOLD 2017 Group D (≥2 exacerbations or ≥1 hospitalization due to exacerbations)* | X | |
| Subgroup analysis by previous treatment | |||
| • ICS use at screening • LABA use at screening • LABA/ICS fixed-dose combination use at screening • LAMA use at screening • LABA/LAMA/ICS use at screening |
X | ||
| X | |||
| X | |||
| X | |||
| X | |||
| Analysis according to HCRU* | |||
| • Requiring treatment with systemic corticosteroids and antibiotics • Requiring treatment with antibiotics • Requiring treatment with systemic corticosteroids alone • Requiring hospitalization • Rehospitalizations within 30 days after a severe exacerbation |
X | ||
| X | |||
| X | |||
| X | |||
| X | |||
| Time to first moderate/severe exacerbation | Subgroup analysis by exacerbation history in the previous year | ||
| • 1 and ≥2 moderate/severe exacerbations • GOLD 2017 Group D (≥2 exacerbations or ≥1 hospitalization due to exacerbations) |
X | ||
| X | |||
| Treatment with antibiotics and/or systemic corticosteroids | Pattern of treatment with antibiotics and/or systemic corticosteroids in patients with ≥2 exacerbations and ≥3 exacerbations during the treatment period | X | |
| COPD deaths | Number and percentage | X |
Note:
Analysis performed only for rate of moderate/severe exacerbations.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; HCRU, health care resource use; ICS, inhaled corticosteroid; LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist.
Participants
Patients aged ≥40 years with a post-bronchodilator forced expiratory volume in 1 second (FEV1) ≥25% and <60% predicted, a documented history of ≥1 COPD exacerbation (for which they received treatment with systemic corticosteroids and/or antibiotics) in the previous 12 months, and mMRC dyspnea scale grade ≥2 were included in the FLAME study. Full inclusion and exclusion criteria have been described previously.6
Definitions of exacerbations
Exacerbations, defined according to Anthonisen criteria, were categorized as mild (worsening of symptoms for >2 consecutive days and not treated with systemic corticosteroids and/or antibiotics), moderate (treated with systemic corticosteroids and/or antibiotics) or severe (requiring hospitalization [or an emergency room visit of >24 hours] in addition to treatment with systemic corticosteroids and/or antibiotics). Worsening of symptoms was captured in an electronic diary that alerted patients and physicians to the presence of an exacerbation. The current analysis focuses on moderate or severe exacerbations. The results of subgroup analysis of the primary outcome (all exacerbations) of the FLAME study have been reported previously.6
Analyses of moderate or severe exacerbations
The rate of and time to first moderate or severe exacerbation with IND/GLY versus SFC was evaluated in different subgroups of symptomatic patients based on the history of exacerbations in the previous year: 1 exacerbation, ≥2 exacerbations, and ≥2 exacerbations or at least 1 hospitalization due to an exacerbation (GOLD 2017 Group D). The rate of exacerbations in the patient subgroups was also analyzed according to pre-recruitment treatment categories (these categories were not mutually exclusive): LAMA, LABA, ICS, LABA/ICS and LAMA/LABA/ICS.
Moderate or severe exacerbations according to HCRU, that is, exacerbations requiring treatment with systemic corticosteroids, antibiotics, or both, and those requiring hospitalization were analyzed and reported. Additionally, the pattern of treatment with antibiotics and/or systemic corticosteroids was evaluated in patients with ≥2 and ≥3 exacerbations during the treatment period.
Statistical analysis
The statistical methods used to analyze the endpoints have been described previously.7 The present analysis used the same approach for all the statistical analyses. The analyses were performed on the full analysis set, which included all randomized patients who received at least 1 dose of the study drug and did not have major violations in compliance with Good Clinical Practice guidelines before unblinding. The number of exacerbations during the treatment period was analyzed using a negative binomial model including terms for treatment such as baseline smoking status, prior ICS use, airflow limitation and region (all as fixed effects), along with baseline total symptom score and COPD exacerbation history as covariates. The analysis was also adjusted for treatment exposure. For subgroup analyses of the exacerbation rate, the same negative binomial model was used with the addition of the subgroup term (if not already included in the model) and a treatment by subgroup interaction. Exacerbation rates were analyzed according to prespecified and post hoc subgroups to assess the consistency of the treatment effect. The time-to-event endpoints were analyzed using a Cox regression model, which included the same terms as the negative binomial model.
Results
Patients
The demographic details of the overall patient population of the FLAME study have been previously reported.6 The mean age of the patients was 64.6 years; 76.1% were male. The mean post-bronchodilator FEV1 was 44.1% of predicted. At screening, 56.3% of patients were using an ICS and 67.1% were using a LABA. Among the patients included in the study, 80.6% (IND/GLY, 1,355; SFC, 1,355) reported 1 exacerbation, 19.3% (IND/GLY, 324; SFC, 325) reported ≥2 exacerbations and 31.1% (IND/GLY, 536; SFC, 511) reported ≥2 exacerbations or an exacerbation that led to hospitalization (ie, GOLD 2017 Group D) in the year before the trial.
Patients with ≥2 exacerbations or ≥1 exacerbation leading to hospitalization had a higher mean COPD assessment test score and a lower St George’s Respiratory Questionnaire for COPD total score. A higher proportion of patients with mMRC dyspnea scale Grade 4 belonged to the GOLD D category, and comparatively more patients were using triple therapy (LABA/LAMA/ICS; Table 2).
Table 2.
Patient demographics and clinical characteristics at baseline based on exacerbation history in the previous year (randomized set)
| Patient characteristics | 1 exacerbation (N=2,710) | ≥2 exacerbations (N=649) | ≥2 exacerbations or ≥1 hospitalization due to exacerbations (N=1,047) |
|---|---|---|---|
| Age (years) | 64.5 (7.8) | 64.7 (7.9) | 64.8 (7.8) |
| Male, n (%) | 2,094 (77.3) | 461 (71.0) | 791 (75.5) |
| Duration of COPD (years) | 7.2 (5.4) | 7.7 (5.5) | 7.2 (5.3) |
| Current smoker, n (%) | 1,088 (40.1) | 243 (37.4) | 403 (38.5) |
| Severity of COPD (GOLD 2015), n (%) | |||
| High risk and more symptoms (GOLD D) | 1,864 (68.8) | 649 (100.0) | 1,047 (100.0) |
| Severity of airflow limitation (GOLD 2011–2014), n (%) | |||
| Moderate (GOLD 2) | 928 (34.2) | 193 (29.7) | 298 (28.5) |
| Severe (GOLD 3) | 1,561 (57.6) | 392 (60.4) | 636 (60.7) |
| Very severe (GOLD 4) | 199 (7.3) | 58 (8.9) | 105 (10.0) |
| Post-bronchodilator FEV1 (L) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) |
| Post-bronchodilator FEV1, % predicted | 44.3 (9.4) | 43.1 (9.6) | 42.6 (9.6) |
| Post-bronchodilator FEV1 reversibility (%) | 22.2 (15.9) | 23.1 (16.4) | 22.6 (16.1) |
| Post-bronchodilator FEV1/FVC (%) | 41.7 (9.7) | 40.8 (10.3) | 41.1 (10.3) |
| COPD medications at screening, n (%) | |||
| ICS alone or in combination | 1,486 (54.8) | 406 (62.6) | 644 (61.5) |
| LAMA alone or in combination | 1,614 (59.6) | 421 (64.9) | 660 (63.0) |
| LABA alone or in combination | 1,794 (66.2) | 462 (71.2) | 722 (69.0) |
| LABA/LAMA/ICS use only | 886 (32.7) | 263 (40.5) | 410 (39.2) |
| LABA/ICS use only | 504 (18.6) | 123 (19.0) | 201 (19.2) |
| LABA/LAMA use only | 276 (10.2) | 62 (9.6) | 88 (8.4) |
| LAMA+ICS use only | 56 (2.1) | 15 (2.3) | 26 (2.5) |
| LABA use only | 128 (4.7) | 14 (2.2) | 23 (2.2) |
| LAMA use only | 396 (14.6) | 81 (12.5) | 136 (13.0) |
| Other | 464 (17.1) | 91 (14.0) | 163 (15.6) |
| SGRQ-C total scorea | 46.5 (15.6) | 50.3 (16.4) | 50.5 (16.3) |
| CAT scoreb | 16.4 (6.9) | 18.0 (7.2) | 17.9 (7.3) |
| mMRC dyspnea scale, n (%) | |||
| Grade 2 | 1,969 (72.7) | 441 (68.0) | 703 (67.1) |
| Grade 3 | 690 (25.5) | 180 (27.7) | 300 (28.7) |
| Grade 4 | 46 (1.7) | 28 (4.3) | 44 (4.2) |
| Rescue medication use (puffs/day) | 3.9 (3.8) | 4.7 (4.3) | 4.6 (4.3) |
| Urine cortisolc (ng/mL) | 15.6 (23.3) | 17.5 (18.9) | 16.4 (17.1) |
Notes: Data are presented as mean (SD) unless otherwise specified;
On a scale of 0–100, with higher scores indicating worse health status;
On a scale of 0–40, with higher scores indicating worse health status;
Twenty-four hour urine cortisol measured in a total of 535 patients (IND/GLY 266, and SFC 269).
Abbreviations: CAT, COPD assessment test; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council; SGRQ-C, St George’s Respiratory Questionnaire for COPD.
Moderate or severe exacerbations by prior history of exacerbation(s) and previous treatment
Exacerbation rates
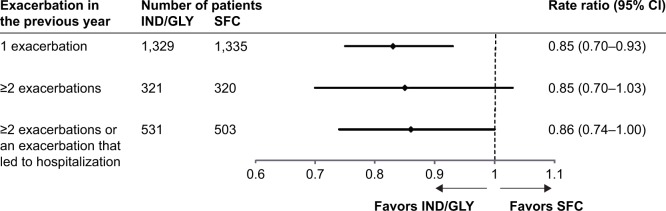
IND/GLY consistently reduced the rate of moderate or severe exacerbations independent of exacerbation history (Figure 1). IND/GLY reduced the rate of moderate or severe exacerbations compared with SFC in patients with a history of 1 exacerbation in the previous year (rate ratio [RR]: 0.83; 95% CI: 0.75–0.93). IND/GLY also numerically reduced the rate of moderate or severe exacerbations by 14%–15% versus SFC in patients with ≥2 exacerbations in the previous year (RR: 0.85: 95% CI: 0.70–1.03) and in those meeting the 2017 GOLD Group D criteria (history of ≥2 exacerbations or at least 1 hospitalization due to exacerbations in the previous year) (RR: 0.86; 95% CI: 0.74–1.00).
Figure 1.
Annualized rate of moderate or severe COPD exacerbations in different subgroups of patients based on prior exacerbations (full analysis set).
Abbreviations: IND/GLY, indacaterol/glycopyrronium; SFC, salmeterol/fluticasone.
Risk of first exacerbation
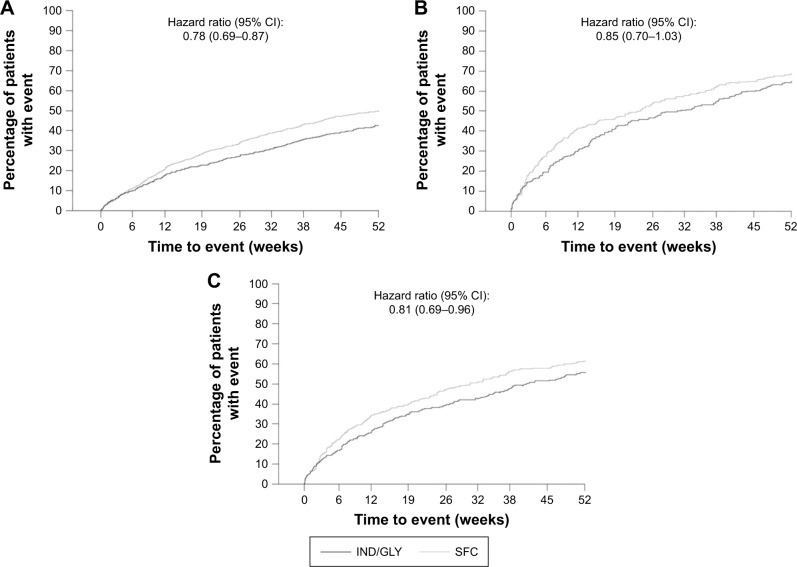
The risk of moderate or severe exacerbations was significantly reduced with IND/GLY compared with SFC in patients with 1 exacerbation (hazard ratio [HR]: 0.78; 95% CI: 0.69–0.87). IND/GLY also numerically reduced the risk of moderate or severe exacerbation in patients with ≥2 exacerbations in the previous year, although this trend did not reach statistical significance (HR: 0.85; 95% CI: 0.70–1.03). A significant reduction in the risk of moderate or severe exacerbations was observed with IND/GLY versus SFC in patients meeting the 2017 GOLD group D criteria (HR: 0.81; 95% CI: 0.69–0.96; Figure 2).
Figure 2.
Kaplan–Meier plot of time to first moderate or severe COPD exacerbation in different subgroups of patients based on prior exacerbations (full analysis set).
Notes: (A) Patients with history of 1 exacerbation in the previous year. (B) Patients with history of ≥2 exacerbations in the previous year. (C) Patients with history of ≥2 exacerbations or at least 1 exacerbation that led to hospitalization in the previous year.
Abbreviations: IND/GLY, indacaterol/glycopyrronium; SFC, salmeterol/fluticasone.
Moderate or severe exacerbations by previous treatment
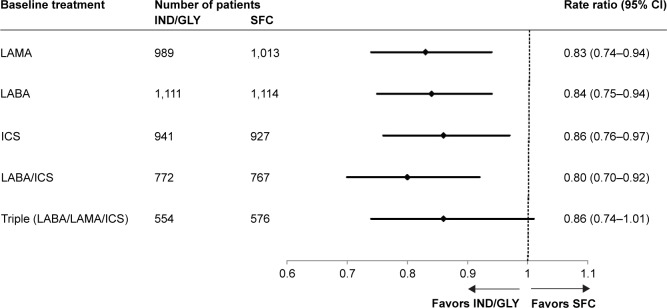
A consistent decrease in the rate of moderate or severe exacerbations was observed with IND/GLY versus SFC in all the groups defined by previous treatment (Figure 3; the groups were not mutually exclusive). A reduction in the exacerbation rate was observed with IND/GLY versus SFC in patients who were receiving LABA/ICS (RR: 0.80; 95% CI: 0.70–0.92), ICS (RR: 0.86; 95% CI: 0.76–0.97), or triple therapy (numerical trend: RR: 0.86; 95% CI: 0.74–1.01) prior to recruitment.
Figure 3.
Annualized rate of moderate or severe COPD exacerbations based on previous treatment (full analysis set).
Abbreviations: ICS, inhaled corticosteroid; IND/GLY, indacaterol/glycopyrronium; LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist; SFC, salmeterol/fluticasone.
Moderate or severe exacerbations according to health care resource use
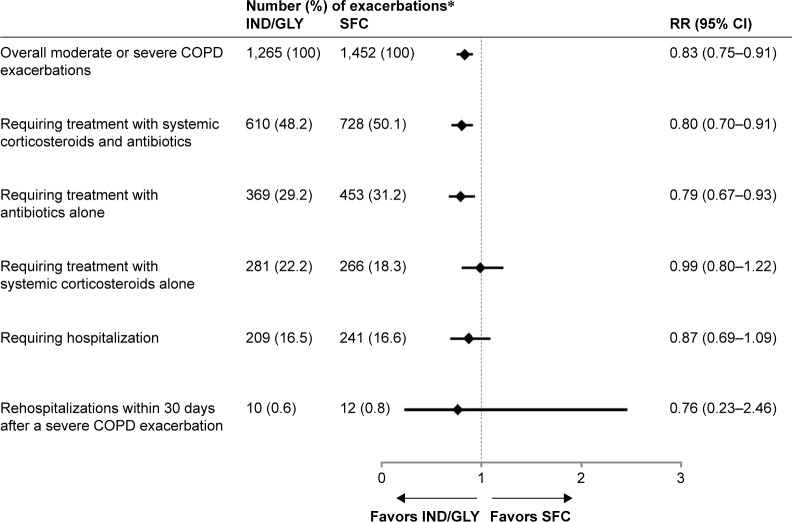
The number of patients and annualized RR of moderate or severe COPD exacerbations according to HCRU are summarized in Figure 4. A significant reduction was observed in the rate of moderate or severe exacerbations, requiring treatment with both systemic corticosteroids and antibiotics in patients treated with IND/GLY compared with those treated with SFC (RR: 0.80; 95% CI: 0.70–0.91). A significant reduction was also observed in the rate of moderate or severe exacerbations, requiring treatment with antibiotics alone in patients treated with IND/GLY compared with those treated with SFC (RR: 0.79; 95% CI: 0.67–0.93). The rate of moderate or severe exacerbations requiring treatment with systemic corticosteroids alone was similar between the treatment groups (RR: 0.99; 95% CI: 0.80–1.22).
Figure 4.
Number and RR of moderate or severe COPD exacerbations according to HCRU with IND/GLY versus SFC during the treatment period.
Notes: *If an exacerbation satisfies multiple criteria (eg, required treatment with a medication and later required hospitalization), then the event is counted in each category (row) satisfied. Thus, the percentages do not add up to 100%.
Abbreviations: HCRU, health care resource utilization; IND/GLY, indacaterol/glycopyrronium; RR, rate ratio; SFC, salmeterol/fluticasone.
During the study period, the management of patients with ≥2 exacerbations with antibiotics, systemic corticosteroids or both presented a significant variation from the first event to the next event. The most consistent pattern was use of both antibiotics and corticosteroids (Figure S1).
Discussion
The FLAME study demonstrated the superiority of IND/GLY over SFC in preventing exacerbations in symptomatic patients with moderate to very severe COPD and a history of previous exacerbations. The analyses reported in this paper further demonstrate a consistent beneficial effect of IND/GLY versus SFC on moderate or severe exacerbations, independent of prior exacerbation history or previous treatment. This consistent beneficial effect was also observed in moderate or severe exacerbations requiring treatment with antibiotics with or without systemic corticosteroids, whereas a similar effect of the 2 treatments was observed on exacerbations treated with systemic corticosteroids alone.
There are different clinical phenotypes of high-risk COPD patients, with some at risk owing to airflow limitation and/or prior exacerbation history and some at risk because of comorbidities.8 Therapeutic approaches based on different patient phenotypes may offer personalized management of the disease.9,10 Prior history of exacerbations has been shown to be a risk factor for future exacerbations,11 and thus exacerbation history might affect exacerbations during the course of the study. Additionally, treatment regimens used at baseline may affect treatment effectiveness due to potential withdrawal effects.12,13 The fact that IND/GLY was more efficacious than SFC, independent of the prior exacerbation history and treatment, adds value to positioning this dual bronchodilator as a preferred treatment option to LABA/ICS for COPD exacerbation in patients. Prevention of COPD exacerbations can have an important impact on the course of disease progression.3 Suissa et al identified the importance of delaying subsequent exacerbations, with each recurrence worsening the course of the disease and increasing the risk of subsequent exacerbations.14 With a window of opportunity available for COPD exacerbation after the first hospitalization, it is important that patients receive optimal treatment at the very earliest opportunity to maximize clinical benefit. The reduction in the rate of moderate or severe exacerbations with IND/GLY versus SFC was maintained in patients with ≥2 exacerbations in the previous year, as well as in patients with either ≥2 exacerbations or ≥1 hospitalization for an exacerbation. As a result of the smaller number of patients, some of the subgroup results were not statistically significant, although the magnitude of exacerbation reduction was consistent with that seen in the overall study population (~15%–20%). Admittedly, hospitalization history is dependent on the availability of resources in different health care systems (eg, availability of hospital-at-home, day care facilities, and availability of hospital beds or presence of specialists in emergency departments) and this needs to be considered in all relevant analyses; however, in our analysis, we have used this definition as proposed in the current GOLD recommendations. However, the magnitude of reduction in these patient groups was consistent with that seen in the overall study population, suggesting that the benefits apply equally to these higher risk groups. In addition, interaction testing did not reveal any significant influence of exacerbation history on treatment effects (data not shown).
In the INSPIRE study, SFC demonstrated a quantitatively similar impact on COPD exacerbations as tiotropium; however, SFC reduced the rate of exacerbations treated with systemic corticosteroids, while tiotropium reduced the rate of those treated with antibiotics.15 Analyses from the SUMMIT study demonstrated that LABA/ICS combination reduced exacerbations treated with oral corticosteroids (with or without antibiotics) when compared with placebo or LABA, but not those treated with antibiotics alone.16 In the FLAME study population, there were significant reductions with IND/GLY versus SFC in the rate of moderate or severe exacerbations requiring antibiotics with or without corticosteroids; however, the annualized rate of moderate or severe exacerbations requiring systemic corticosteroids alone (~20% of the exacerbations) was similar between the treatment groups. This finding suggests that the two treatments have differential effects on different types of exacerbations according to physicians’ clinical judgement; however, the similar efficacy of IND/GLY and SFC also on the corticosteroid-treated events provides reassurance on the role of this treatment for exacerbation prevention. Our data add a novel piece of information that advances our understanding of the role of inhaled combinations on different types of events according to health care resource use. In order to elucidate the drivers of the different choice of treatment for exacerbations, these data need to be evaluated in combination with the appropriate characterization of exacerbations, according to etiology and baseline symptoms at the onset of each event in further analyses.
Although reports have shown additional beneficial effects of the use of a dual bronchodilator versus LABA/ICS,17–19 there are only limited clinical studies of LABA/LAMA combinations (FLAME [IND/GLY versus SFC]6 and SPARK [IND/GLY versus GLY]20) that evaluated exacerbations as the primary endpoint. Two recent meta-analysis of the pooled efficacy and safety data from the trials of fixed-dose combinations of LABA/LAMA reported that the only LABA/LAMA that significantly reduced moderate/severe exacerbation rate compared with LABA/ICS was IND/GLY versus SFC based on the available studies.21,22 These results strengthen the body of evidence that LABA/LAMA is superior to LABA/ICS for the majority of COPD patients on several COPD outcomes, whereas exacerbation prevention is rather specific to the comparison of IND/GLY versus SFC based on the available published data.
The mechanism of reduction of the risk of exacerbations with LABA/LAMA is not well understood, and in a recent review, Beeh et al comprehensively proposed 4 possible mechanisms of how dual long-acting bronchodilators prevent COPD exacerbations.23 These mechanisms include decrease in hyperinflation and mechanical stress, modulation of mucus production and mucociliary clearance, improvement in symptoms fluctuation and severity and some potential direct and indirect anti-inflammatory effects.23 In different pivotal clinical trials of the IGNITE program, IND/GLY demonstrated a significant reduction in hyperinflation and a significant improvement in symptoms versus placebo.21 Although a head-to-head comparison of IND/GLY and SFC for the reduction of hyperinflation was not performed, there are a few reports of LABA/LAMA showing significant improvement in spirometry measures related to hyperinflation (inspiratory capacity).23 Nonetheless, IND/GLY showed significant improvements in dyspnea, health status and rescue medication use versus SFC.6,24,25 Therefore, the likely mechanism for the reduction of exacerbations with LABA/LAMA combinations is the improved spirometric outcome, resulting in a decrease in hyperinflation and an improvement in symptoms, most notably dyspnea.
Some limitations of these results must be acknowledged. These are secondary analyses of the FLAME study and therefore certain conclusions from these data should be interpreted with caution. Some subgroup analyses dealt with small sample sizes or few events. Nonetheless, trends consistently favored IND/GLY across multiple analyses, thus providing reassurance that the beneficial effects of IND/GLY compared with SFC are not limited to specific subpopulation(s). Moreover, the results refer specifically to the comparison of IND/GLY 110/50 μg once daily versus SFC 50/500 μg twice daily administered through the specific devices (Breezhaler® versus Diskus®/Accuhaler®) in the population of symptomatic patients with moderate to very severe COPD and a history of exacerbations in the previous year included in the FLAME study. Whether these results are applicable to other combinations of LABA/LAMA versus LABA/ICS needs to be evaluated in specifically designed trials.
Conclusion
These analyses extend the results of the FLAME study by demonstrating the superiority of a LABA/LAMA combination, IND/GLY, over a LABA/ICS combination, SFC, for all exacerbation outcomes across a heterogeneous patient population at risk of COPD exacerbations. Differences in the relative preventive effects of the tested treatments by exacerbation treatment-defined subtypes need further investigation.
Acknowledgments
The FLAME study was sponsored by Novartis Pharma AG. The authors would like to thank patients and staff at the participating centers. The authors also thank Sinéad Flannery, Chiranjit Ghosh and Santanu Sannigrahi (professional medical writers; Novartis) for assistance in the preparation of this manuscript. Writing support was funded by the study sponsor. All the authors vouch for the accuracy and completeness of the data.
Footnotes
Disclosure
CFV has received grant funding, honoraria for lectures and/or participation in advisory boards from AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GSK, Grifols, Menarini, Mundipharma, Novartis, Teva, and Cipla. KRC reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants from Baxter, grants and personal fees from CSL Behring, grants and personal fees from Grifols, grants from GlaxoSmithKline, grants and personal fees from Sanofi, grants and personal fees from Genentech, grants and personal fees from Kamada, grants from Amgen, grants and personal fees from Roche, grants and personal fees from Novartis, personal fees from Merck, personal fees from CIHR-GSK Research Chair in Respiratory Health Care Delivery, UHN, during the conduct of the study. NR has received grants and personal fees from Boehringer Ingelheim, Novartis, Pfizer; personal fees from Teva, GSK, AstraZeneca, Chiesi, Mundipharma, Cipla, Sanofi, Sandoz, 3M, and Zambon, outside the submitted work. JV reports personal fees from GlaxoSmithKline, Chiesi pharmaceuticals, Boehringer-Ingelheim, Novartis, Almirall, AstraZeneca, Bioxydyn, Ferring, outside the submitted work. MM has received speaker fees from Boehringer Ingelheim, Chiesi, Cipla, Menarini, Grifols and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Gebro Pharma, CLS Behring, Novartis and Grifols. JAW reports grants from GSK, grants from Johnson and Johnson, other from Novartis, other from Boehringer Ingelheim, other from Astra Zeneca, other from GSK, grants from GSK, grants from Astra Zeneca, grants from Boehringer Ingelheim, grants from Novartis, outside the submitted work. CT, DB, RF, FP and KK are employees and shareholders of Novartis Pharma AG. PO is an employee of Novartis AB and a Novartis shareholder. The authors report no other conflicts of interest in this work.
Author contributions
JAW, CV, KRC, MM, NR, JV, CT, DB, RF, FP, PO and KK all made substantial contributions to the conception and design of the analysis, and interpretation of data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All authors provided their final approval to the version to be published. The authors are accountable for the accuracy and integrity of this work.
References
- 1.Adeloye D, Chua S, Lee C, et al. Global Health Epidemiology Reference G Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Campos JL, Agusti A. Heterogeneity of chronic obstructive pulmonary disease exacerbations: a two-axes classification proposal. Lancet Respir Med. 2015;3(9):729–734. doi: 10.1016/S2213-2600(15)00242-8. [DOI] [PubMed] [Google Scholar]
- 6.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 7.Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med. 2017;195(9):1189–1197. doi: 10.1164/rccm.201701-0193OC. [DOI] [PubMed] [Google Scholar]
- 8.Agusti A, Hurd S, Jones P, et al. FAQs about the GOLD 2011 assessment proposal of COPD: a comparative analysis of four different cohorts. Eur Respir J. 2013;42(5):1391–1401. doi: 10.1183/09031936.00036513. [DOI] [PubMed] [Google Scholar]
- 9.McDonald VM, Higgins I, Wood LG, Gibson PG. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax. 2013;68(7):691–694. doi: 10.1136/thoraxjnl-2012-202646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miravitlles M, Soler-Cataluna JJ, Calle M, Soriano JB. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J. 2013;41(6):1252–1256. doi: 10.1183/09031936.00118912. [DOI] [PubMed] [Google Scholar]
- 11.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 12.Suissa S, Ariel A. The FULFIL trial. Am J Respir Crit Care Med. 2018;197(4):542. doi: 10.1164/rccm.201708-1578LE. [DOI] [PubMed] [Google Scholar]
- 13.Lipson DA, Barnacle H, Birk R, et al. Reply to Suissa and Ariel: the FULFIL trial. Am J Respir Crit Care Med. 2018;197(4):542–543. doi: 10.1164/rccm.201709-1831LE. [DOI] [PubMed] [Google Scholar]
- 14.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wedzicha JA, Calverley PM, Seemungal TA, et al. INSPIRE Investigators The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 16.Martinez FJ, Vestbo J, Anderson JA, et al. SUMMIT Investigators Effect of fluticasone furoate and vilanterol on exacerbations of chronic obstructive pulmonary disease in patients with moderate airflow obstruction. Am J Respir Crit Care Med. 2017;195(7):881–888. doi: 10.1164/rccm.201607-1421OC. [DOI] [PubMed] [Google Scholar]
- 17.Beeh KM, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat® is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler® (ENERGITO® study) Int J Chron Obstruct Pulmon Dis. 2016;11:193–205. doi: 10.2147/COPD.S95055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109(7):870–881. doi: 10.1016/j.rmed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48(4):1030–1039. doi: 10.1183/13993003.00216-2016. [DOI] [PubMed] [Google Scholar]
- 20.Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi: 10.2147/COPD.S130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD) Cochrane Database Syst Rev. 2017;2:CD012066. doi: 10.1002/14651858.CD012066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beeh KM, Burgel PR, Franssen FME, et al. How do dual long-acting bronchodilators prevent exacerbations of chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2017;196(2):139–149. doi: 10.1164/rccm.201609-1794CI. [DOI] [PubMed] [Google Scholar]
- 24.Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026. doi: 10.2147/COPD.S84436. [DOI] [PMC free article] [PubMed] [Google Scholar]