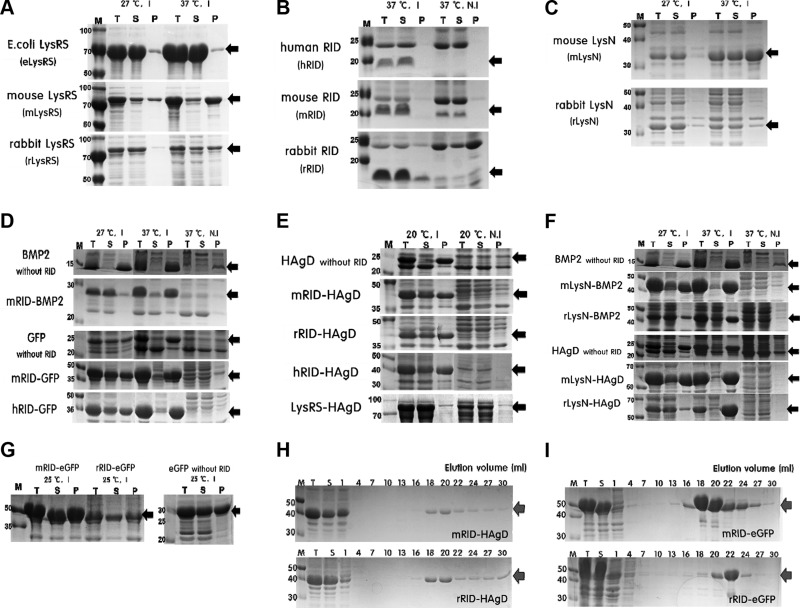
Figure 2.
Soluble expression and purification of recombinant proteins in E. coli. A) A comparison of LysRS proteins derived from E. coli, mice, and rabbits in terms of their solubility and expression levels. I, induction; N.I, noninduction; T, total extract; S, soluble fraction; P, pellet fraction. Black arrows, target proteins. B) A comparison of RIDs derived from humans, mice, and rabbits in terms of their solubility and expression levels. All of the RID proteins were expressed solubly at 37°C. C) Soluble expression data on each LysN (mouse and rabbit origin). D) Soluble expression data on RID-fused proteins. E) Expression profiles of recombinant proteins RID-HAgD and eLysRS-HAgD and HAgD without RID. F) Soluble expression of LysN-fused proteins. G) Soluble expression of RID-eGFP and eGFP without RID, a control fusion protein. H) SDS-PAGE data on purification of RID-HAgD proteins that were expressed at 20°C. I) Purification data on mRID-eGFP and rRID-eGFP proteins expressed at 25°C as a control protein. Red arrows, purified target proteins. All of the data were obtained in duplicate.