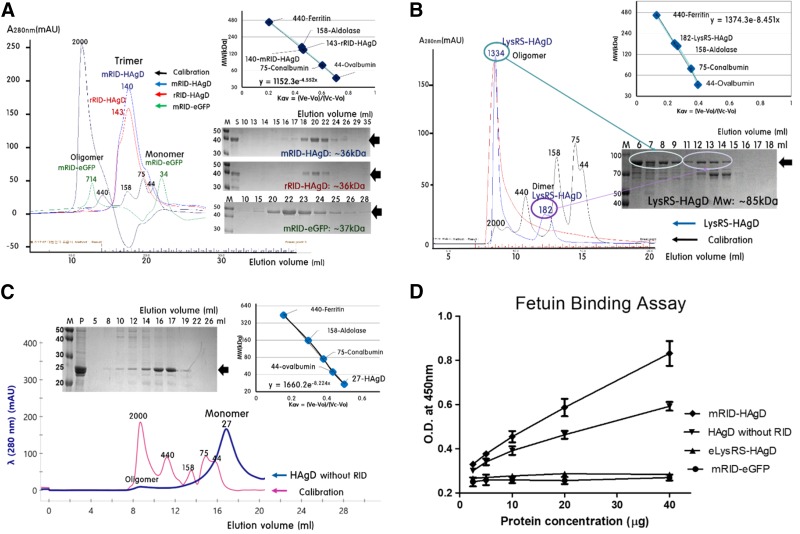
Figure 3.
Determination of the oligomeric status of fusion antigens by gel-filtration chromatography and a fetuin-binding assay. A) mRID-HAgD and rRID-HAgD were eluted in trimeric form on the basis of Kav, followed by analysis of RID-HAgD by SDS-PAGE. mRID-eGFP, as a control, was eluted as a mixture of oligomeric (30%) and monomeric (70%; estimated at 34.2 kDa) forms. B) LysRS-HAgD, as a control, was eluted as a mixture of oligomeric (60%) and dimeric (40%; estimated as 182 kDa) forms. C) The refolded HAgD without RID was eluted predominantly in monomeric form (80%; estimated at 25.6 kDa). D) The glycoprotein (fetuin), which contains terminal sialic acid residues as a receptor for HA, was adsorbed to a microplate and analyzed for binding to mRID-HAgD, HAgD without RID, and eLysRS-HAgD. The mRID-eGFP protein served as a negative control. All of the data were obtained in duplicate.