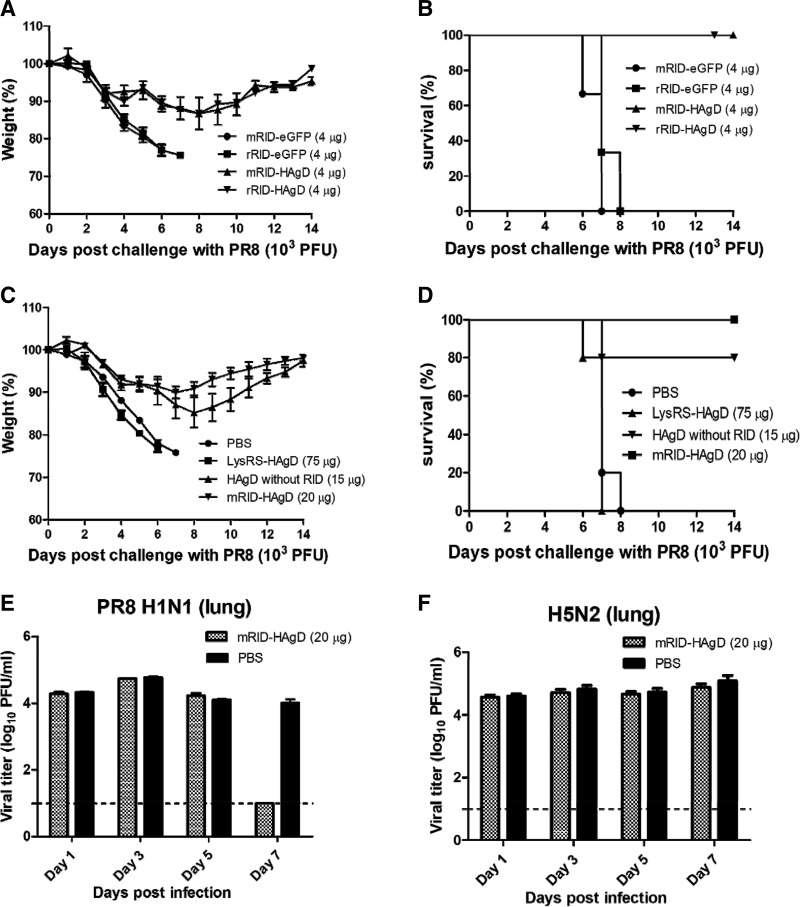
Figure 8.
Protective efficacy of the recombinant vaccine against a PR8 (H1N1) virus challenge. A, B) The mice immunized (intraperitoneally) with 4 μg RID-HAgD (n = 6) or RID-eGFP control (n = 6), along with the Alum adjuvant, were challenged with 1 times LD50 of the PR8 (H1N1) virus (103 PFUs), and the body weight changes (A) and survival rates (B) of the infected mice were monitored daily. Immunization with the RID-eGFP control (n = 6) failed to provide protection, and all of these mice (n = 6) succumbed within 7 dpc. C, D) The mice vaccinated intraperitoneally with 20 μg of the mRID-HAgD immunogen (n = 5) or mock infected (PBS control; n = 5) were challenged with 1 times LD50 of the PR8 (H1N1) virus (103 PFUs), and the body weight changes (C) and survival rates (D) of the infected mice were monitored daily. The mice immunized with 75 μg LysRS-HAgD (n = 5) or 15 μg of the HAgD without RID (n = 5) were also challenged with 1 times LD50 of the PR8 (H1N1) virus (103 PFUs), and body weight changes of the infected mice were monitored daily (C). Immunization with eLysRS-HAgD or PBS failed to provide protection, and all of these mice (n = 5) succumbed within 7 dpc. Immunization with the HAgD without RID provided partial protection, and only one mouse succumbed within 7 dpc (D). E, F) Replication of the challenge virus in the lungs of the immunized mice. The titers of challenge virus in the lungs were measured by a viral plaque assay. Dashed lines denote a detection limit, 1.0. Error bars indicate the sd of each cohort.