Figure 1.
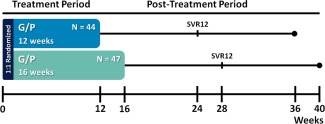
MAGELLAN‐1, Part 2 study design. Patients were randomized 1:1 and stratified by HCV GT (1 or 4) and past treatment experience (NS5A inhibitor‐naïve or ‐experienced) to receive either 12 or 16 weeks of once‐daily G/P (300 mg/120 mg). All patients had past VF, virological failure to at least one HCV treatment regimen containing an approved NS3/4A protease and/or NS5A inhibitor allowed by study protocol. Patients were followed for 24 weeks posttreatment to monitor safety and SVR.
