Figure 2.
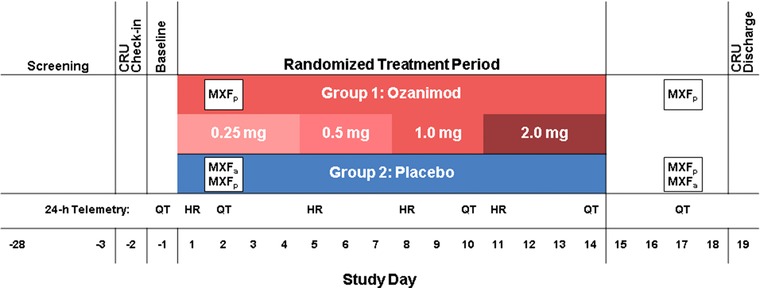
Study design. The ozanimod group also received moxifloxacin placebo on days 2 and 17. Within the placebo group, subjects were randomized (1:1) to receive a single dose of moxifloxacin 400 mg or placebo on days 2 and 17. CRU indicates clinical research unit; HR, 24‐hour cardiac telemetry for heart‐rate analysis; MXF, moxifloxacin (positive control); MXFa, MXF active (400 mg); MXFp, MXF placebo; QT, 24‐hour telemetry for QT assessment.
