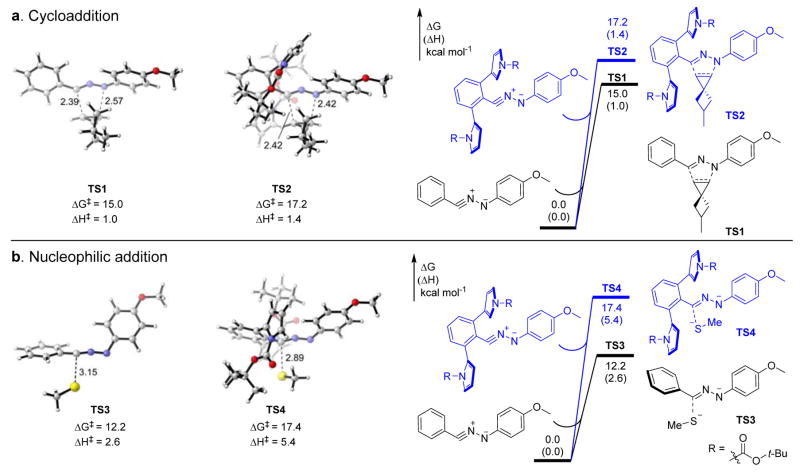
Figure 1. Computational studies of the steric shielding effect of the ortho N-Boc-pyrrole substituents.
Computed transition states and activation energies involving nitrile imine-1 and -26 for (a) 1,3-dipolar cycloaddition with 5-methyl-spiro[2.3]hex-1-ene, and (b) nucleophilic addition with methyl thiolate, respectively. DFT calculations were performed at the ωB97x-D/6-311++G(d,p)/SMD(Water) level of theory using geometries optimized by B3LYP-D3/6-31+G(d)/SMD(Water). R = N-Boc. The distances shown are in Å, and energies are in kcal/mol.