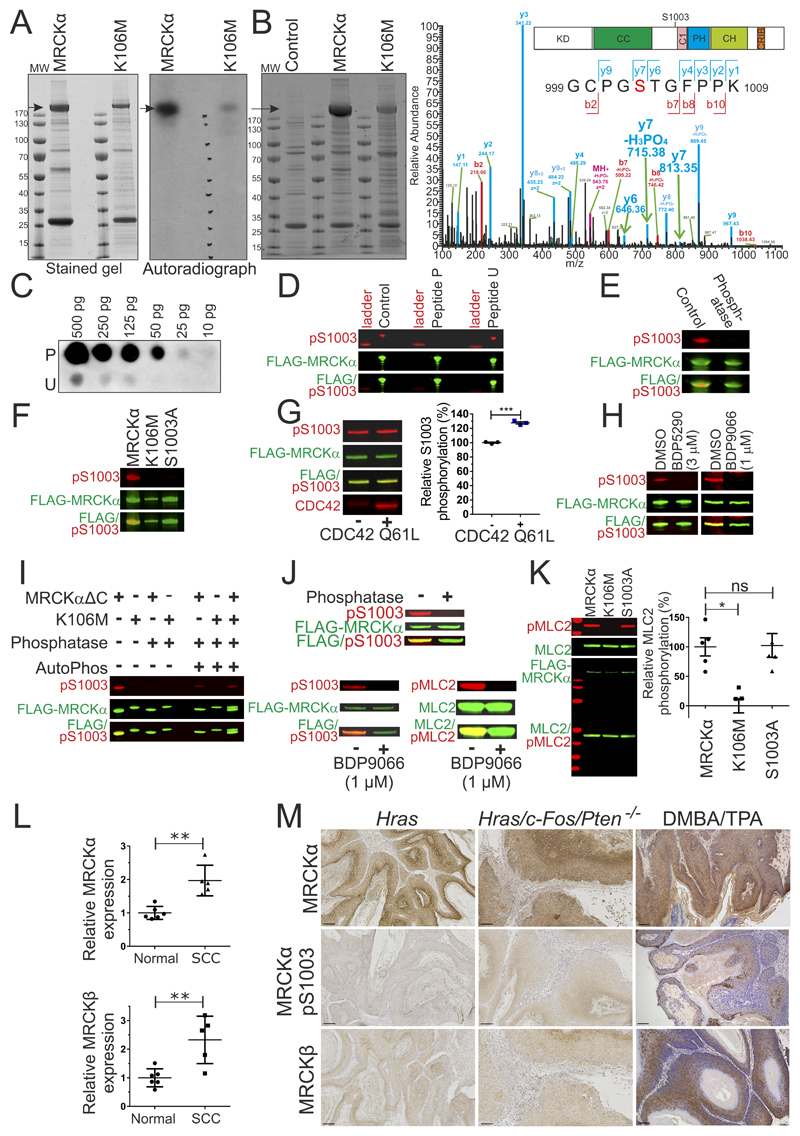
Figure 5. MRCKα S1003 autophosphorylation as a biomarker of activity.
(A) Stained SDS-PAGE gel (left) of FLAG immunoprecipitated FLAG-MRCKα and FLAG-MRCKα K106M after in vitro 32P incorporation assay. Arrow indicates MRCKα. Gel autoradiograph (right) shows 32P labelling of MRCKα. MW = molecular weight. (B). Stained SDS-PAGE gel (left) of FLAG-immunoprecipitates from control HEK293 cells, or cells transfected with pEF-FLAG-MRCKα or pEF-FLAG-MRCKα K106M. Arrow indicates MRCKα. MW = molecular weight. Higher energy collision dissociation (HCD) MS/MS fragmentation spectra (right) of MRCKα tryptic peptide 999-1009 containing S1003 (red lettering) in MRCKα or MRCKα K106M (phosphorylation inferred from y7, y7-H3PO4 and y6 signals indicated with green arrows). See Supplemental Table 8 for theoretical y and b fragmentation ion series masses. Diagram of MRCKα protein domains (inset) illustrating location of S1003. (KD = kinase domain, CC = Coiled-coiled domain, C1 = C1 diacylglycerol binding domain, PH = Pleckstrin homology domain, CH = Citron Homology domain, CRIB = Cdc42/Rac interactive binding motif). (C). Dot blot of MRCKα peptides (10 to 500 pg) containing phosphorylated S1003 (peptide P) or unphosphorylated S1003 (peptide U), stained with pS1003 antibody (D). Western blots of FLAG-immunoprecipitated FLAG-MRCKα stained with untreated pS1003 antibody, or pS1003 antibody pre-incubated with phosphorylated S1003 peptide P or unphosphorylated peptide U (E). Western blots of FLAG-immunoprecipitated FLAG-MRCKα, incubated in phosphatase buffer without (control) or with lambda phosphatase (phosphatase) (F). Western blot of FLAG-immunoprecipitated FLAG-MRCKα, FLAG-MRCKα K106M or FLAG-MRCKα S1003A. (G). Western blot (left) of FLAG-immunoprecipitated FLAG-MRCKα incubated in kinase buffer in the absence or presence of recombinant CDC42 Q61L protein. Relative S1003 phosphorylation revealed that CDC42 significantly increased autophosphorylation. Results shown are means ± SEM from 3 experiments. Student’s t-test (*** = p<0.001). (H). Western blots of HEK293 cells expressing FLAG-MRCKα treated with DMSO, 3 µM BDP5290 or 1 µM BDP9066 (I). Western blots of FLAG immunoprecipitated FLAG-MRCKαΔC or FLAG-MRCKα K106M untreated or treated with lambda phosphatase, or treated with lambda phosphatase and then incubated in kinase buffer to allow autophosphorylation (AutoPhos). (J) Western blots of FLAG-immunoprecipitated FLAG-MRCKα, untreated or treated with lambda phosphatase (upper panel). Incubation with kinase buffer resulted in autophosphorylation (pS1003; lower left panel) and recombinant MLC2 substrate phosphorylation (pMLC2; lower right panel) (K). Western blots of FLAG-immunoprecipitated FLAG-MRCKα, FLAG-MRCKα K106M or FLAG-MRCKα S1003A incubated in vitro with recombinant MLC2 in a kinase buffer (left). Relative MLC2 phosphorylation by each kinase revealed that K106M significantly reduced activity, while S1003A was not different from wild-type. Results shown are means ± SEM from 5 independent experiments. One way Kruskal-Wallis ANOVA followed by post-hoc Dunn’s multiple comparison (* = p<0.05). (L) Relative MRCKα (upper) and MRCKβ (lower) expression was significantly higher in cutaneous SCC relative to normal human skin determined by microarray analysis (45). Results shown are means ± SD from 6 normal skin and 5 SCC patients. Two-tailed Mann-Whitney test of significance (** = p<0.01). (M) Immunohistochemistry of MRCKα, MRCKα pS1003 or MRCKβ in benign papillomas from mice expressing oncogenic Hras in epidermal keratinocytes, or in well-differentiated squamous cell carcinomas resulting from Hras/c-Fos/Pten-/- genetic modifications or DMBA/TPA chemical carcinogenesis. Scale bars = 100 µm.