Abstract
Background
Working memory (WM) is often assessed with serial order tests such as repeating digits backward. In prior dementia research using the Backward Digit Span Test (BDT), only aggregate test performance was examined.
Objective
The current research tallied primacy/recency effects, out-of-sequence transposition errors, perseverations, and omissions to assess WM deficits in patients with mild cognitive impairment (MCI).
Methods
Memory clinic patients (n = 66) were classified into three groups: single domain amnestic MCI (aMCI), combined mixed domain/dysexecutive MCI (mixed/dys MCI), and non-MCI where patients did not meet criteria for MCI. Serial order/WM ability was assessed by asking participants to repeat 7 trials of five digits backwards. Serial order position accuracy, transposition errors, perseverations, and omission errors were tallied.
Results
A 3 (group) × 5 (serial position) repeated measures ANOVA yielded a significant group × trial interaction. Follow-up analyses found attenuation of the recency effect for mixed/dys MCI patients. Mixed/dys MCI patients scored lower than non-MCI patients for serial position 3 (p < 0.003) serial position 4 (p < 0.002); and lower than both group for serial position 5 (recency; p < 0.002). Mixed/dys MCI patients also produced more transposition errors than both groups (p < 0.010); and more omissions (p < 0.020), and perseverations errors (p < 0.018) than non-MCI patients.
Conclusions
The attenuation of a recency effect using serial order parameters obtained from the BDT may provide a useful operational definition as well as additional diagnostic information regarding working memory deficits in MCI.
Keywords: Boston process approach, digit span, executive functions, mild cognitive impairment, serial order, working memory
INTRODUCTION
Mild cognitive impairment (MCI) is generally believed to be a prodrome that often results in the emergence of dementia syndromes such as Alzheimer’s disease (AD) and is considered to be a useful construct to identify patients who are potentially at risk for developing a dementing illness. Diagnostic criteria for MCI include the subjective complaint of memory and/or other neurocognitive problems, along with relative preservation of instrumental activities of daily living, in conjunction with objective evidence documenting a decline in memory and/or other neurocognitive abilities [1–3]. Patients diagnosed with MCI can be classified as presenting with single versus multiple domain subtypes [4, 5].
The importance of investigating MCI subtypes revolves around several clinical as well as theoretical issues including a greater understanding of conversion to dementia and a clearer appreciation of the brain-behavior relationships that underlie MCI syndromes. For example, past empirical findings suggest greater reliability for the eventual emergence of dementia for amnestic and multi-domain MCI as compared to single domain dysexecutive MCI [6–10]. Recent research has also shown that when mixed/dysexecutive MCI patients are defined using neuropsychological criteria there tends to be faster progression to dementia than other groups [11, 12]. Second, the investigation of MCI subtypes provides an opportunity to investigate the neurocognitive constructs underlying brain-behavior relationships associated with MCI. For example, Libon et al. [13] have shown that patients with amnestic MCI differ from other MCI subtypes on a variety of linguistic as well as memory parameters obtained from a serial list learning test. Eppig et al. [14] found that impairment on executive tests produced by mixed and dysexecutive MCI patients were similar and remarkable in that performance deteriorated as a function of time to completion and/or test epoch. The emergence of this striking negative slope, or steep temporal gradient as described by Fuster [15], suggests difficulty maintaining mental set. Eppig et al. [14] suggested that steeper temporal gradients may provide a useful heuristic for understanding brain-behavior relationships that underlie impairment on executive tests in patients with MCI. In addition to unique patterns of behavior obtained from neuropsychological measures, patients with dysexecutive MCI have also been distinguished from other MCI subtypes using and neuroimaging parameters [4, 16–18].
Tests that examine serial order recall, such as the Backward Digit Span Test (BDT) [19, 20] as described by Lamar et al. have been used to assess working memory deficits in both dementia and MCI. The BDT has previously been analyzed to generate two gross aggregate measures including total any recall – tallied as the total percent recall of digits regardless of their correct serial order and believed to provide a measure of auditory span; and total serial order recall – tallied as the total percent recall of digits in the exact serial order and believed to provide a measure of working memory and the capacity for mental manipulation. Lamar et al. [19, 20] found that total serial order recall was able to differentiate patients with AD versus vascular dementia who presented with MRI evidence of subcortical white matter alterations. Moreover, reduced aggregate serial order recall was associated with greater MRI evidence of left-sided frontal and posterior parietal white matter alterations.
Karl Lashley [21] was among the first researchers to address what he termed The Problem of Serial Order1. Subsequent experimental research assessing serial order, such as asking participants to repeat digits backward, has been used to operationally-define a number of specific parameters related to working memory including primacy and recency effects and the generation of specific patterns of errors (see [22] for a complete review]. First, prior research has consistently shown that when young, healthy participants are asked to repeat numbers backward, recency effects are enhanced [23, 24]. Second, errors commonly seen in serial order research in younger adults most often include out-of-sequence, transposition errors, i.e., a response that is recalled in the wrong serial position. Third, transposition errors can be expressed in terms of a transposition gradient that measures the degree of displacement between their correct position and incorrect response position [22]. Fourth, serial ordering research has also identified non-transposition errors, termed items errors that include omissions and perseverations. Fifth, prior serial order research has suggested that item errors are far less frequent than transposition errors.
In the current research, the BDT [19, 20] was used to assess serial order recall in patients with suspected MCI. As noted above, prior research using the BDT tallied only aggregate serial order test performance. In the current study, detailed analyses of the five components of serial order recall described above were undertaken including the calculation of primacy/recency effects; an analysis of transposition errors; the calculation of transposition displacement; and the occurrence of item errors. The primary goal of the current research is to assess how these five benchmark parameters measuring serial order recall that have been well-researched in younger research participants can differentiate between MCI subgroups. On the basis of prior research [11, 12, 14] where both dysexecutive and mixed MCI patients presented with lower test scores on executive tests, as well as greater difficulty in sustaining mental set on executive tests, i.e., a steeper temporal gradient [15], our primary prediction is that mixed/dysexecutive MCI patients will present with an attenuated recency effect, more total transposition errors along with greater transposition displacement, and greater numbers of item errors compared to other MCI groups.
METHODS
Participants
Participants studied in the current research (n = 66) were recruited from Rowan University, New Jersey Institute for Successful Aging, Memory Assessment Program (MAP). All MAP patients underwent a comprehensive neuropsychological evaluation and were also examined by a social worker and a board certified geriatric psychiatrist. An MRI study of the brain and appropriate serum blood tests were obtained to evaluate for reversible causes of dementia. A clinical diagnosis was determined for each patient at an interdisciplinary team conference. Participants diagnosed with MCI presented with subjective cognitive complaints and/or evidence of cognitive impairment relative to age and education, preservation of general functional abilities, and the absence of dementia. Participants were excluded if there was any history of head injury, substance abuse, or major psychiatric disorders, including major depression, epilepsy, B12, folate, or thyroid deficiency. For all participants, a knowledgeable family member was available to provide information regarding functional status. This study was approved by the Rowan University institutional review board with consent obtained consistent with the Declaration of Helsinki.
Neuropsychological assessment
The neuropsychological protocol used to classify MCI subtype assessed three domains of cognition: executive control, naming/lexical access, and declarative memory. From this protocol, nine parameters, three from each neurocognitive domain, were used to classify MCI subtype as described below. All test scores were expressed as z-scores derived from normative data. We acknowledge that other neuropsychological tests/domains of cognitive functioning could have been used. The rationale for using the protocol described above was based on prior research showing that these tests are able to illustrate key neurocognitive constructs and differentiate between MCI subtypes (see [11–13]).
Executive control
This cognitive domain was assessed with three tests including The Boston Revision of the Wechsler Memory Scale-Mental Control subtest [25], the letter fluency test [26]; and the Trail Making Test-Part B [27]. The dependent variable for the Mental Control subtest was the total non-automatized accuracy index (see [25] for full details). The dependent variables obtained from the letter fluency test and Trail Making Test-Part B were the demographically-corrected scores provided by Heaton et al. [28].
Lexical access/language
This domain was also assessed with three tests, including the 60-item version of the Boston Naming Test [29]; a test of semantic (‘animals’) fluency where participants were asked to produce as many names of animals in 60 s excluding perseverations and extra-category intrusion responses [30]; and the Wechsler Adult Intelligence Scale-III Similarities subtest [31]. The dependent variables for the Boston Naming Test and ‘animal’ fluency tests were obtained from Heaton et al. [28]. The dependent variable obtained from the WAIS-III Similarities subtest was the age-corrected scale score.
Memory and learning
This cognitive domain was assessed with the 9-word California Verbal Learning Test-Mental Status test [32]. This test was scored and administered using standard instructions. Three CVLT-short form variables were used in the current research including total immediate free recall, delayed free recall, and the delayed recognition measure. Executive control, lexical access/language, and memory and learning index scores were computed by averaging performance among the three tests in each domain.
Determination of mild cognitive impairment subtypes
Single and multi-domain MCI
Jak et al. [33] criteria were used to determine MCI subtype. Single domain MCI syndromes were diagnosed when participants scored >1.0 standard deviation below normative expectations on any of two of the three measures within a single cognitive domain. Mixed MCI syndromes were diagnosed when participants scored >1.0 standard deviation below normative expectations on any of two of the three measures within two or more cognitive domains. On the basis of these procedures, 15 patients were diagnosed with single domain amnes-tic MCI (aMCI), 3 patients were diagnosed with single domain dysexecutive MCI, and 15 were diagnosed with mixed or multi-domain MCI. Because of the small number of dysexecutive MCI patients a combined mixed/dysexecutive (mixed/dys) MCI subgroup (n = 18) was constructed. This decision was made on the basis of prior research [11, 12, 14] where mixed/dysexecutive patients presented with similar patterns of impairment on executive tests.
Non-MCI group
Among the patients who presented for clinical evaluation, 33 did not meet Jak et al. [33] criteria for MCI. Some of these patients (n = 17) performed such that all nine neuropsychological parameters were above 1 SD. A second group of patients (n = 16) not meeting criteria for MCI presented with some, but very little, cognitive impairment such that 13 patients produced tests scores where only 1 of the 9 neuropsychological parameters was below the 1 SD cut-off; and 3 patients produced neuropsychological test scores where only two neuropsychological parameters across different domains of cognitive functioning were below 1 SD. When these groups not meet criteria for MCI were compared on the serial order outcome variables described below no differences were found. For this reason, these patients were combined into a single group and labeled as presenting with non-MCI.
Neuropsychological index scores
When the memory test index was assessed between-group aMCI patients scored lower compared to other groups (aMCI versus non-MCI; p < 0.001, aMCI versus mixed/dys MCI; p < 0.005); and mixed/dys MCI patients scored lower than non-MCI patients (p < 0.003). Between-group analyses for the executive test index found that mixed/dys MCI patients scored lower than other groups (mixed/dys MCI versus non-MCI; p < 0.001, mixed/dys MCI versus aMCI; p < 0.003; respectively). Similarly, on the language test index mixed/dys MCI patients scored lower than other groups (mixed/dys MCI versus non-MCI; p < 0.001, mixed/dys MCI versus aMCI; p < 0.021; respectively; Table 1). Thus, patients with aMCI presented with circumscribed impairment on memory tests. The predominant neuropsychological impairment among mixed/dys MCI patients revolved around lower scores on executive and language tests as compared to other groups.
Table 1.
Demographic and clinical information: means and standard deviations
| non-MCI (n = 33) |
aMCI (n = 15) |
mixed/dys MCI (n = 18) |
significance | |
|---|---|---|---|---|
| Age | 77.27 (6.26) | 76.20 (6.48) | 77.88 (5.46) | ns |
| Education | 14.63 (3.00) | 14.20 (2.67) | 13.50 (2.95) | ns |
| MMSE | 27.69 (1.75) | 26.73 (2.21) | 26.44 (1.58) | ns |
| WRAT-IV Reading subtest | 116.30 (18.60) | 116.46 (13.37) | 110.61 (16.39) | ns |
| IADL abilities | 14.90 (2.44) | 13.38 (3.52) | 14.23 (2.70) | ns |
| Geriatric Depression Scale | 3.63 (2.52) | 3.06 (2.71) | 4.11 (3.12) | ns |
| Executive Index (z-scores) |
–0.12 (0.36) | –0.37 (0.49) | –1.02 (0.77) | mixed/dys MCI < non-MCI; p < 0.001 mixed/dys MCI < aMCI; p < 0.003 |
| Naming/Lexical access Index (z-scores) |
–0.10 (0.50) | –0.27 (0.60) | –0.91 (0.91) | mixed/dys MCI < non-MCI; p < 0.001 mixed/dys MCI < aMCI; p < 0.021 |
| Memory Index (z-scores) |
–0.04 (0.67) | –1.48 (0.49) | –0.71 (0.78) | aMCI < non-MCI; p < 0.001 aMCI < mixed/dys MCI; p < 0.005 mixed/dys MCI < non-MCI; p < 0.003 |
aMCI, amnestic mild cognitive impairment; mixed/dys MCI, multi-domain/dysexecutive mild cognitive impairment; IADL, instrumental activities of daily living; WRAT-IV, Wide Range Achievement Test-IV; ns, not significant.
The backward digit span test
The BDT is comprised of seven trials of 3-, 4-, and 5-digit span lengths for a total of 21 trials. As described by Lamar et al. [19, 20], 4- and 5-span trials were constructed so that contiguous numbers were placed in strategic positions. Thus, in 4-span trials contiguous numbers were placed in either the first and third or second and fourth digit positions, e.g., 5269 or 1493. For 5-span trials, contiguous numbers were placed in the middle three digit positions, e.g., 16579. This procedure cannot be used for 3-span test trials because of primacy and recency effects. The BDT was administered using Wechsler Adult Intelligence Scale administration procedures except that all 21 test trials were administered regardless of errors that were made. To maximize the assessment of serial order position effects only the seven 5-span trials were used in the current research.
Serial order recall/backward digit span outcome variables
Correct responses and primacy and recency effects
The number of correct responses for the seven 5-span trials was tallied (range 0–35, correct). The total percent correct for each of the five serial order positions was also tallied. Recency recall was defined as the first number heard and participants’ subsequent last response. Primacy recall was determined as the last number heard and participants’ subsequent first response. This terminology regarding primacy and recency effects is standard in serial order position research [22, p. 5; 23–24].
Transposition errors and transposition gradient
Total instances of out-of-sequence or transposition errors were tallied. Transposition gradients were also expressed vis-á-vis the degree of displacement in relation to their correct serial position. Anticipation transposition errors were scored using a negative displacement value because they occurred in advance or ahead of their correct serial position. Postponement transposition errors were scored using a positive displacement value because they occurred after their correct serial position. Correctly recalled test items were assigned a value of zero to reflect the absence of any displacement. For each group transposition gradients were plotted in terms of their displacement [22].
Item errors
A variety of non-out-of-sequence or item errors were tallied as described below. Between-trial perseverations were tallied when a number from the preceding two trials was pulled into the current response; within-trial perseverations were tallied when a number within a trial is repeated. Between-trial capture errors were scored when a number from either of the preceding two trials is pulled into the current response creating a contiguous, automatized string of digits; within-trial capture errors were scored when number(s) within the same trial were incorrectly repeated, also creating a contiguous string. Omissions were tallied when the patient responded with less than the number of digits administered. Because of the low frequency of some of these errors all perseveration and capture errors were summed and labeled dysexecutive errors. Omissions and total dysexecutive errors were summed to create a total item error score.
Statistical analyses
The number of correct responses was analyzed using 1-way analysis of variance (ANOVA). Primacy and recency effects were examined using a 3 (group) × 5 (serial position) repeated measures ANOVA. Recency effects were also assessed within-group by comparing recency recall (5th digit recalled, i.e., first number administered, last number recall) versus primacy recall (1st digit recalled, i.e., last number administered, first number recalled). The effect of group for total transposition errors, total anticipation and postponement transposition errors was assessed with multivariate analysis of variance (MANOVA). Because of restriction of range transposition gradient/displacement was expressed by averaging all anticipation and postponement transposition errors to create two separate indices and was assessed with a single MANOVA. Omission, dysexecutive, and total item errors were assessed with a single MANOVA. Finally, the relationship between BDT test performance, Mini-Mental State Examination (MMSE), and overall neuropsychological functioning was assessed with a regression analysis where 5-span serial order recall was the dependent variable. MMSE test performance was entered along with the three neuropsychological domain index scores calculated as described above. Significance was set at p < 0.050. The Bonferroni correction was used for all post-hoc analyses.
RESULTS
Demographic characteristics and ANY/SERIAL recall
Table 1 lists demographic and clinical information. No between-group differences were found for age, education, the MMSE [34], depression assessed with the Geriatric Depression Scale, projected pre-morbid general intellectual abilities as assessed with the Wide Range Achievement Test Reading subtest-IV (WRAT-IV), or Instrumental Activities of Daily Living [35]. Table 2 lists total aggregate ANY order and SERIAL order recall for the three groups. A multivariate analysis of variance (MANOVA) found a significant effect for group (Hotelling’s Trace F[4, 122.00] = 4.01, p < 0.004, hp2 = 0.116). No differences were found for ANY order recall; however, differences were found for SERIAL order recall (F[2, 63] = 7.68; p < 0.001, hp2 = 0.196) where post-hoc (Bonferroni) analyses found that mixed/dys MCI patients scored lower compared to non-MCI patients (p < 0.001) and aMCI patients (p < 0.043).
Table 2.
Serial order recall: means and standard deviations
| non-MCI (n = 33) |
aMCI (n = 15) |
mixed/dys MCI (n = 18) |
Significance | |
|---|---|---|---|---|
| any order recall | 94.37 (5.82) | 92.00 (4.73) | 89.68 (9.20) | ns |
| serial order recall | 81.81 (17.28) | 77.52 (10.46) | 62.85 (19.32) | mixed/dys MCI< non-MCI; p < 0.001 |
| mixed/dys MCI< aMCI; p < 0.043 | ||||
| correct responses (0 – 35) | 29.03 (4.99) | 26.13 (4.94) | 22.22 (6.60) | mixed/dys MCI < non-MCI; p < 0.001 |
| backward serial position 1 (primacy) | 97.83 (6.30) | 99.04 (3.68) | 95.23 (8.48) | ns |
| backward serial position 2 | 91.34 (13.33) | 93.33 (10.61) | 84.12 (13.76) | ns |
| backward serial position 3 | 79.22 (19.26) | 70.47 (21.91) | 56.34 (27.92) | mixed/dys MCI < non-MCI; p < 0.003 |
| backward serial position 4 | 67.27 (22.32) | 55.23 (20.81) | 40.47 (33.68) | mixed/dys MCI < non-MCI; p < 0.002 |
| backward serial position 5 (recency) | 83.11 (19.70) | 69.52 (16.96) | 38.09 (37.31) | mixed/dys MCI < non-MCI; p < 0.001 mixed/dys MCI < aMCI; p < 0.002 |
aMCI, amnestic mild cognitive impairment; mixed/dys MCI, multi-domain/dysexecutive mild cognitive impairment; ns, not significant.
Correct responses and primacy/recency effects
One way ANOVA for the number of correct responses (range 0–35) was significant (F[2, 63] = 9.08, p < 0.001) and found that mixed/dys MCI patients recalled fewer correct responses only compared to non-MCI patients (p < 0.001). The 3 group × 5 serial order position repeated measured ANOVA yielded a significant serial order position by group interaction (F[8, 118] = 4.57, p < 0.001; hp2 = 0.237). Follow-up ANOVAs found differences for middle serial position 3 (F[2, 63] = 6.03; p < 0.004), serial order position 4 (F[2, 63] = 6.43; p < 0.003), and recency serial position 5; F[2, 63] = 18.57; p < 0.001) positions. Post-hoc (Bonferroni) comparisons found that mixed/dys MCI patients recalled less information than non-MCI patients for serial order position 3 (p < 0.003) and serial order position 4 (p < 0.002); and that mixed/dys MCI patients recalled less information compared to both groups for serial position 5 (recency; mixed/dys MCI versus non-MCI; p < 0.001; mixed/dys MCI versus aMCI; p < 0.002; Table 3; Fig. 1).
Table 3.
Backward digit span errors: means and standard deviations
| Transposition Errors | non-MCI (n = 33) |
aMCI (n = 15) |
mixed/dys MCI (n = 18) |
significance |
|---|---|---|---|---|
| total transposition errors | 3.75 (3.48) | 4.80 (3.12) | 9.05 (5.29) | mixed/dys MCI > non-MCI; p < 0.001 mixed/dys MCI > aMCI; p < 0.01 |
| total anticipation transposition errors | 2.24 (2.12) | 2.93 (1.75) | 5.11 (3.30) | mixed/dys MCI > non-MCI; p < 0.001 mixed/dys MCI > aMCI; p < 0.039 |
| total postponement transposition errors | 1.51 (1.58) | 1.86 (1.59) | 3.94 (2.43) | mixed/dys MCI > non-MCI; p < 0.001 mixed/dys MCI > aMCI; p < 0.006 |
| average anticipation displacement | 0.56 (0.53) | 0.68 (0.44) | 1.27 (0.82) | mixed/dys MC I > non-MCI; p < 0.001 mixed/dys MCI > aMCI; p < 0.021 |
| average postponement displacement | 0.37 (0.39) | 0.46 (0.39) | 0.98 (0.60) | mixed/dys MCI > non-MCI; p < 0.001 mixed/dys MCI > aMCI; p < 0.006 |
| total omissions | 0.09 (0.39) | 0.46 (0.63) | 0.61 (0.91) | mixed/dys MCI > non-MCI; p < 0.022 |
| total dysexecutive errors | 2.78 (2.25) | 3.73 (2.25) | 4.94 (3.26) | mixed/dys MCI > non-MCI; p < 0.017 |
| total errors | 2.87 (2.45) | 4.20 (2.36) | 5.55 (3.51) | mixed/dys MCI > non-MCI; p < 0.005 |
aMCI, amnestic mild cognitive impairment; mixed/dys MCI, multi-domain/dysexecutive mild cognitive impairment; ns, not significant.
Fig. 1.
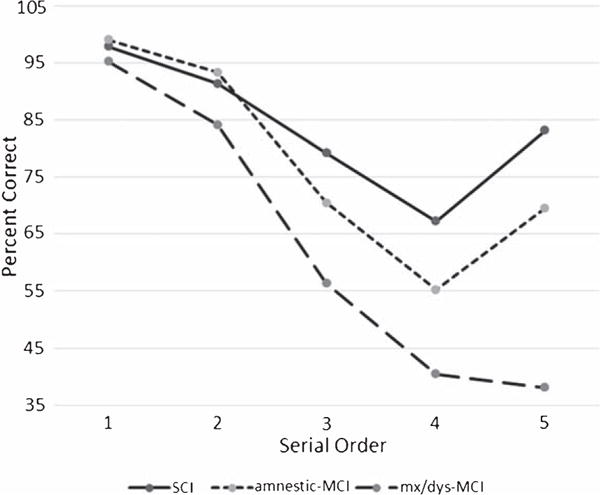
Percent Backward Serial Order Recall.
Total transpositions and transposition gradient
A multivariate analysis of variance (MANOVA) found a significant effect for group (Hotelling’s Trace F[4, 122] = 5.38; p < 0.001, hp2 = 0.150). Univariate group effects were found for total transposition errors, (F[2, 63] = 010.53; p < 0.001, hp2 = 0.251), total anticipation transposition errors (F[2, 63] = 8.20; p < 0.001, hp2 = 0.207), and total postponement transposition errors (F[2, 63] = 10.41; p < 0.001, hp2 = 0.248). Post-hoc (Bonferroni) comparisons found that mixed/dys MCI patients made more total transposition errors compared to non-MCI (p < 0.001) and aMCI (p < 0.010) patients; more anticipation transposition errors than non-MCI patients (p < 0.001) and aMCI (p < 0.039) patients; and more postponement transposition errors than non-MCI (p 0.001) and aMCI (p < 0.006) patients.
Transposition gradients are displayed in Fig. 2. The MANOVA measuring the effect of group for averaged anticipation and postponement transposition displacement was significant (Hotelling’s Trace F[4, 122] = 5.30; p < 0.001, hp2 = 0.148). Group effects were obtained for both anticipation (F[2, 63] = 8.32, p < 0.001, hp2 = 0.209) and postponement (F[2, 63] = 10.41, p < 0.001, hp2 = 0.248) displacement. Post-hoc (Bonferroni) analyses found that mixed/dys MCI patients generated greater anticipation (mixed/dys MCI versus non-MCI, p < 0.001; mixed/dys MCI versus aMCI, p < 0.021) and postponement (mixed/dys MCI versus non-MCI, p < 0.001; mixed/dys MCI versus aMCI, p < 0.006; Fig. 2) displacement than other groups.
Fig. 2.
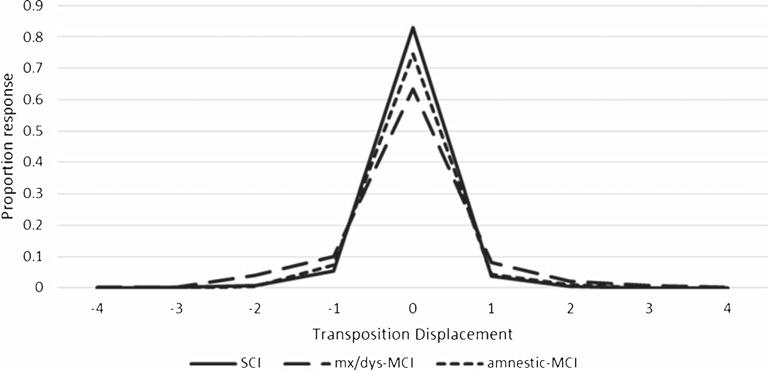
Transposition Gradient.
Item errors
The MANOVA that assessed for group differences for total omissions, total dysexecutive errors, and total errors was significant (F[4, 120] = 3.40, p < 0.011, hp2 = 0.102). The effect for group was found for omissions (F[2, 62] = 4.36, p < 0.017 hp2 = 0.124), dysexecutive errors (F[2, 62] = 4.10, p < 0.021 hp2p = 0.117), and total errors (F[2, 62] = 5.48, p < 0.006 hp2 = 0.150). Post-hoc (Bonferroni) analyses found greater numbers of omissions, dysexecutive, and total errors for mixed/dys MCI patients compared to non-MCI patients (omissions – p < 0.022; dysexecutive errors – p < 0.017; total errors – p < 0.005). Three within-group tests were conducted to compare total transpositions versus total item errors. There was no difference between non-MCI and aMCI patients suggesting that these patient groups made equal numbers of transposition and item errors. However, mixed/dys MCI patient generate almost twice as many total transposition errors compared to item errors (t[17] = 3.18, p < 0.005).
Regression analysis
The relationship between BDT performance, MMSE, and overall neuropsychological functioning was assessed with a regression analysis where 5-span serial order recall was the dependent variable. MMSE test performance was entered along with the three neuropsychological domain index scores. This analysis was significant with only the executive control index entering the model (r = 0.593, R2 = 0.351, df = 4, 61, p < 0.001, beta = 0.519, p < 0.001).
DISCUSSION
In prior research Eppig et al. [14] found that both mixed and dysexecutive MCI patients exhibited derailed or worse performance as a function of time or test epoch compared to normal controls and amnestic MCI patients. These findings were interpreted to reflect greater impairment in sustaining mental set, behavior that is consistent with Fuster’s [15] model of temporal gradients. The current research sought additional information regarding working memory difficulty in MCI with the analysis of five well-researched parameters measuring serial order recall using the BDT. Serial order recall using both forward and backward digit span and related tasks has been thoroughly researched in young, healthy participants. However, to our knowledge there are no studies that have examined how and/or if impairment in serial order recall may provide important information regarding working memory deficits in MCI.
Consistent with the goal and primary prediction of the current research the overall pattern of behavior described above found that parameters measuring serial order recall were able to differentiate mixed/dys MCI patients from non-MCI and aMCI patients; however, there were no differences when aMCI and non-MCI patients were compared. This finding is interesting to the extent that derailed serial order recall as described above might provide a means to characterize and operationally-define working memory deficits in MCI. As described above, mixed/dys MCI patients recalled fewer correct responses than other groups. More interesting were the striking between- and within- group serial order position effects. As shown in Fig. 1, both non-MCI and aMCI patients produced the expected recency effect. By contrast, and consistent with our prediction, there was an attenuated recency effect in the mixed/dys MCI group. Indeed, similar to the data described by Eppig et al. [14] a relentless negative slope characterized performance in this group suggesting striking working memory deficits.
With respect to the analysis of transposition errors, non-MCI and aMCI patients did not differ; however, mixed/dys MCI patients generated more total, anticipation, and postponement transposition errors compared to other groups. Mixed/dys MCI patients also produced greater numbers of item errors including more omissions, dysexecutive errors, and total non-transposition errors.
In sum, the results of prior serial order research conducted with young, healthy participants seen in the laboratory [22] comports well with the patterns of performance obtained from MCI patients seen in the clinic as described above. A notable exception revolves around the generation of item errors. Past serial order research conducted with younger participants has generally reported greater numbers of transposition compared to item errors. However, in the current research, non-MCI and aMCI patients produced equal numbers of transposition errors and item errors; and, mixed/dys MCI patients made almost twice as many transposition errors than item errors. Despite this exception, the data obtained in the current research suggests that serial order recall as measured with the BDT appears to provide an excellent means to operationally-define severity of working memory deficits in patients with suspected MCI. A limitation of the current research is the lack of imaging data that might be used to gain further insight into serial order recall.
Mixed/dys MCI patients scored lower on executive as well as other tests. This raises the question of whether the attenuated recency effect as described above and other indications of impaired serial order recall observed in the mixed/dys group are, in fact, specifically related to executive impairment. The regression analysis described above found that 5-span serial order recall was, in fact, associated only with executive test performance. Nonetheless, more research is required to address these issues.
The current study is consistent with prior research demonstrating reduced serial order recall in other patient groups well-known to present with executive impairment. Zokaei et al. [36] studied patients with Parkinson’s disease (PD) and found that reduced digit span backward was correlated with impaired serial order recall for spatial orientation. Warden et al. [37] examined PD patients with dementia, MCI, and no MCI and found that digit span backward test performance was able to differentiate PD-no MCI patients from other groups. Klekociuk and Summers [38] found lower digit backward test performance in their sample of mixed MCI compared to aMCI patients. Hampstead et al. [39] assessed serial order using a letter span task and found that vascular dementia patients associated with subcortical white matter alterations (i.e., leukoaraiosis) produced more dysexecutive errors compared to patients with AD suggesting greater working memory deficits in vascular dementia as compared to AD.
Performance on serial order recall tasks, such as backward digit span, has been used to support constructs consistent with Competitive Queuing (CQ) models of working memory [22, 40–42]. CQ models of working memory generally posit the existence of two interconnected layers (1) an excitatory parallel planning mechanism; and (2) an inhibitory competitive choice/response suppression mechanism. Parallel planning is the mechanism that is believed to be responsible for the initial excitatory activation of all elements in the sequence to be recalled. In parallel planning neural nodes corresponding to each memoranda to be recalled are activated; however, the strength of activation for each node varies depending on task parameters. After initial activation, competitive choice/response suppression governs the actual output and order of recall. The item with the greatest activation is selected for recall. Following competitive choice, an inhibitory feedback system, known as response suppression, is believed to remove items from the planning layer so the next strongest activated item can be recalled. This process continues iteratively until all items are recalled [22, 43, 44]. Recent electrophysiological research provides some evidence for CQ-related constructs. For example, when single cell activity from frontal regions were obtained from macaque monkeys, behavior similar to transposition errors were found in relation to diminished neural activity prior to a successfully executed movement [45–47]. In humans, Agam and colleagues [48, 49] found decreased event related potentials associated with behavior similar to transposition errors.
There is now substantial research demonstrating greater and more widespread gray and white matter compromise in mixed MCI compared to single domain MCI subtypes [50–54]. These neuroradiological findings are related to the current research in that both white matter and anterior and posterior gray matter integrity have been linked to performance on tests that assess working memory and executive control in normal controls and MCI. For example, in a sample of normal control participants Sasson et al. [55] found that executive test performance was correlated with diffusion tensor imaging parameters involving both the superior longitudinal fasciculus and uncinate fasciculus. Bettcher et al. [56] also studied a large sample of normal control participants with a wide array of executive tests including digit span backward. MRI parameters including both global gray matter and white matter structures such as the cingulum and corpus callosum contributed to intact performance on executive tests. Libon et al. [57] showed that greater whole brain leukoaraiosis was associated with reduced aggregate BDT serial order recall in a sample of MCI participants.
These observations are consistent with data reported by Lamar et al. [20] showing that greater anterior and posterior left-hemisphere leukoaraiosis was related to impaired aggregate BDT serial order recall in patients with dementia thought to correspond to arcuate and longitudinal fasciculi. Interestingly, Lamar et al. [20] have pointed out that visuospatial processing has been linked with successful number sequencing and that this cognitive operation is associated with a bidirectional network involving the parietal cortex [58]. Moreover, past research examining backward digit test performance suggests that visuospatial imagery may be a strategy used to facilitate performance [59]. It is possible that the attenuated recency effect in our mixed/dys MCI group, as well as the production of greater numbers of omissions and dysexecutive errors may be associated with a neurocognitive network involving inferior parietal and occipito-temporal regions combined with a disruption between posterior and frontal regions [58]. However, this notion is speculative and must be the subject of prospective research. Also, there is considerable documentation that white matter alterations result in slow information processing speed in patients with MCI [60]. Impaired information processing speed could have negatively affected performance on the BDT among our mixed/dys MCI patients. This is another area for further research. Coupling patterns of performance regarding serial order recall as expressed with tests such as the BDT with neuroradiological parameters may provide a means for assessing both the presence and severity of WM deficits in MCI.
The current study has several strengths including the analysis of process and errors to better understand serial order neurocognitive constructs [61, 62] and the use of objective criteria to classify MCI and non-MCI subtypes. However, several limitations must be acknowledged including a modest sample size. First, our definition of MCI was limited to three neurocognitive domains. Other areas of cognitive functioning should be investigated in conjunction with serial order recall. Second, as noted above, no neuroradiological information was available for correlation with cognitive performance. Despite these limitations, our findings provide evidence that an analysis of serial order recall can reliably differentiate between MCI subtypes. Future work should investigate whether parameters measuring serial order recall obtained with the BDT can be dissociated using neuroradiological parameters in order to provide additional information regarding working memory deficits in MCI.
Acknowledgments
Supported by K24-AG046373 (ALJ), K01-AG049164 (TJH), K12-HD043483 (KAG, TJH), and Paul B. Beeson Career Development Award in Aging K23-AG045966 (KAG). This research was supported, in part, by funding from the NIH – K2CX 000938 (KJB); and K24 AG026431 and R01 AG04 9810 (MWB).
Footnotes
Karl Lashley (1951) viewed the mechanism(s) that link individual or discrete behavior into complex cognitive operations to be poorly understood and dubbed this conundrum ‘The Problem of Serial Order’. Lashley’s seminal paper on this topic was part of the famous Hixon Symposium. The Hixon Symposium was convened at the California Institute of Technology in September, 1948 by Lloyd Jeffress of the University of Texas and subsequently published in 1951. This symposium focused on a wide number of cerebral mechanisms that underlie brain and cognition. The symposium was composed of a distinguished group of neuroscientists such as Ward Halstead, Heinrich Kluver, Wolfgang Kohler, as well as Karl Lashley. The topics discussed were diverse and far reaching. Howard Gardner (1985) traces the origins of what has been termed “The Cognitive Revolution” to the papers delivered at this meeting.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17–0555r2).
References
- 1.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jaqust WJ, Peterson RC, Snyder PJ, Carrillo MC, Thies B, Phelp CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC, Morris JD. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 3.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, B¨ackman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Dujin C, Visser P, Petersen RC. Mild cognitive impairment – beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 4.Delano-Wood L, Abeles N, Bondi MW, Sacco J, Jak AJ, Libon DJ, Bozoki A. Heterogeneity in mild cognitive impairment: Differences in neuropsychological profile and associated white matter lesion pathology. J Int Neuropsychol Soc. 2009;15:906–914. doi: 10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libon DJ, Xie SX, Eppig J, Wicas G, Lamar M, Lippa C, Bettcher BM, Price CC, Giovannetti T, Swenson R, Wambach DM. The heterogeneity of mild cognitive impairment: A neuropsychological analysis. J Inl Neuropsychol Soc. 2010;16:84–93. doi: 10.1017/S1355617709990993. [DOI] [PubMed] [Google Scholar]
- 6.Huey ED, Manly JJ, Tang MX, Schupf N, Brickman AM, Manoochehri M, Mez J, DeCarli C, Devanand DP, Mayeux R. Course and etiology of dysexecutive MCI in a community sample. Alzheimers Dement. 2013;9:632–639. doi: 10.1016/j.jalz.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessen E, Reinvang I, Eliassen CF, Nordlund A, Gjerstad L, Fladby T, Wallin A. The combination of dysexecutive and amnestic deficits strongly predicts conversion to dementia in young mild cognitive impairment patients: A report from the Gothenburg-Oslo MCI Study. Dem Geriat Cogn Dis-Extra. 2014;4:76–85. doi: 10.1159/000360282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JK, Pa J, Boxer AL, Kramer JH, Freeman K, Yaffe K. Baseline predictors of clinical progression among patients with dysexecutive mild cognitive impairment. Dem Geriat Cogn. 2010;30:344–351. doi: 10.1159/000318836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Hu B, Ma X, Xu L. Resting-state whole-brain functional connectivity networks for mci classification using l2-regularized logistic regression. IEEE Trans Nanobiosci. 2015;14:237–247. doi: 10.1109/TNB.2015.2403274. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z, Wu L, Jia J, Han Y. Advances in longitudinal studies of amnestic mild cognitive impairment and Alzheimer’s disease based on multi-modal MRI techniques. Neurosci Bull. 2014;30:198–206. doi: 10.1007/s12264-013-1407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP, for the Alzheimer’s Disease Neuroimaging Initiative Neuropsychological criteria For MCI improves diagnostic precision, biomarker associations and prediction of progression. J Alzheimers Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas KR, Edmonds EC, Delano-Wood L, Bondi MW, for the Alzheimer’s Disease Neuroimaging Initiative Longitudinal trajectories of informant-reported daily functioning in empirically-defined subtypes of mild cognitive impairment. J Int Neuropsychol Soc. 2017;23:521–527. doi: 10.1017/S1355617717000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libon DJ, Bond MW, Price CC, Lamar M, Eppig J, Wambach DM, Nieves C, Delano Wood L, Giovannetti T, Lippa C, Kabasakalian A, Cosentino S, Swenson R, Penney DL. Verbal serial list learning in mild cognitive impairment: A profile analysis of interference, forgetting, and errors. J Intl Neuropsychol Soc. 2011;17:905–914. doi: 10.1017/S1355617711000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eppig J, Wambach DM, Nieves C, Price CC, Lamar M, Delano-Wood L, Giovannetti T, Bettcher BM, Penney DL, Swenson R, Lippa C, Kabasakalian A, Bondi MW, Libon DJ. Dysexecutive functioning in mild cognitive impairment: Derailment in temporal gradients. J Int Neuropsychol Soc. 2012;18:20–28. doi: 10.1017/S1355617711001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuster JM. The Prefrontal Cortex. Fourth. Academic Press; London: 2008. [Google Scholar]
- 16.Chao LL, Pa J, Duarte A, Schuff N, Weiner MW, Kramer JH, Miller BL, Freeman KM, Johnson JK. Patterns of cerebral hypoperfusion in amnestic and dysexecutive MCI. Alzheimer Dis Assoc Dis. 2009;23:245–252. doi: 10.1097/WAD.0b013e318199ff46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delano-Wood L, Abeles N, Sacco JM, Wierenga CE, Horne NR, Bozoki A. Regional white matter pathology in mild cognitive impairment. Stroke. 2008;39:794–799. doi: 10.1161/STROKEAHA.107.502534. [DOI] [PubMed] [Google Scholar]
- 18.Pa J, Boxer A, Chao LL, Gazzaley A, Freeman K, Kramer JH, Miller BL, Weiner MW, Neuhaus J, Johnson JK. Clinical-neuroimaging characteristics of dysexecutive mild cognitive impairment. Ann Neurol. 2009;65:414–423. doi: 10.1002/ana.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamar M, Price CC, Libon DJ, Penney DL, Kaplan E, Grossman M, Heilman KM. Alterations in working memory as a function of leukoaraiosis in dementia. Neuropsychologia. 2007;45:245–254. doi: 10.1016/j.neuropsychologia.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamar M, Catani M, Price CC, Heilman KM, Libon DJ. The impact of region specific leukoaraiosis on working memory deficits in dementia. Neuropsychologia. 2008;46:2597–2601. doi: 10.1016/j.neuropsychologia.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Lashley K. The problem of serial order in behavior. In: Jeffress LA, editor. Cerebral mechanisms in behavior: The Hixon Symposium. Wiley; New York: 1951. pp. 112–146. [Google Scholar]
- 22.Hurlstone MJ, Hitch GJ, Baddeley AD. Memory for serial order across domains: An overview of the literature and directions for future research. Psychol Bull. 2014;140:339–373. doi: 10.1037/a0034221. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JR, Bothell D, Lebiere C, Matessa M. An integrated theory of list memory. J Mem Lang. 1998;38:341–380. [Google Scholar]
- 24.St Clair-Thompson HL, Allen RJ. Are forward and backward recall the same? A dual-task study of digit recall. Mem Cogn. 2013;41:519–532. doi: 10.3758/s13421-012-0277-2. [DOI] [PubMed] [Google Scholar]
- 25.Lamar M, Price CC, Davis KL, Kaplan E, Libon DJ. Capacity to maintain mental set in dementia. Neuropsychologia. 2002;40:435–445. doi: 10.1016/s0028-3932(01)00125-7. [DOI] [PubMed] [Google Scholar]
- 26.Spreen O, Strauss E. Compendium of Neuropsychological Tests. Oxford University Press; New York: 1990. [Google Scholar]
- 27.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Vol. 4. Reitan Neuropsychology; 1985. [Google Scholar]
- 28.Heaton RK, Miller S, Taylor M, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults scoring programs. Psychological Assessment Resources; 2004. [Google Scholar]
- 29.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 30.Carew TG, Lamar M, Cloud BS, Grossman M, Libon DJ. Impairment in category fluency in ischaemic vascular dementia. Neuropsychology. 1997;11:400–412. doi: 10.1037//0894-4105.11.3.400. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. Psychological Corporation; 1997. [Google Scholar]
- 32.Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test-II. Psychology Corporation; New York: 2000. [Google Scholar]
- 33.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriat Psychia. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Lawton MP, Brody E. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 36.Zokaei N, Burnett Heyes S, Gorgoraptis N, Budhdeo S, Husain M. Working memory recall precision is a more sensitive index than span. J Neuropsychol. 2015;9:319–329. doi: 10.1111/jnp.12052. [DOI] [PubMed] [Google Scholar]
- 37.Warden C, Hwang J, Marshall A, Fenesy M, Poston KL. The effects of dopamine on digit span in Parkinson’s disease. J Clin Movement Dis. 2016;3:5. doi: 10.1186/s40734-016-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klekociuk SX, Summers M. Lowered performance in working memory and attentional sub-processes are most prominent in multi-domain amnestic mild cognitive impairment subtypes. Psychogeriatrics. 2014;14:63–71. doi: 10.1111/psyg.12042. [DOI] [PubMed] [Google Scholar]
- 39.Hampstead BM, Libon DJ, Moelter ST, Swirsky-Sacchetti T, Scheffer L, Platek SM, Chute D. Temporal order memory differences in Alzheimer’s disease and vascular dementia. J Clin Expl Neuropsychol. 2010;32:645–654. doi: 10.1080/13803390903418918. [DOI] [PubMed] [Google Scholar]
- 40.Grossberg S. Behavioral contrast in short-term memory: Serial binary memory models or parallel continuous memory models. J Math Psychol. 1978;17:199–219. [Google Scholar]
- 41.Grossberg S. A theory of human memory: Self-organization and performance of sensory motor codes, maps, and plans. In: Rosen R, Snell FM, editors. Progress in Theoretical Biology. Vol. 5. Academic Press; New York: 1978. pp. 233–374. [Google Scholar]
- 42.Houghton G. Current research in natural language generation. Academic Press Professional, Inc; 1990. The problem of serial order: A neural network model of sequence learning and recall; pp. 287–319. [Google Scholar]
- 43.Davelaar EJ, Goshen-Gottstein Y, Ashkenazi A, Haarmann HJ, Usher M. The demise of short-term memory revisited: Empirical and computational investigations of the recency effects. Psychol Rev. 2005;112:3–42. doi: 10.1037/0033-295X.112.1.3. [DOI] [PubMed] [Google Scholar]
- 44.Farrell S, Lewandowsky S. Modelling transposition latencies: Constraints for theories of serial order memory. J Mem Lang. 2004;51:115–135. [Google Scholar]
- 45.Averbeck BB, Chafee MV, Crowe DA, Georgopoulos AP. Parallel processing of serial movements in prefrontal cortex. Proc Natl Acad Sci U S A. 2002;99:13172–13177. doi: 10.1073/pnas.162485599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Averbeck BB, Chafee MV, Crowe DA, Georgopoulos AP. Neural activity in prefrontal cortex during copying geometrical shapes: I. Single cells encode shape, sequence, and metric parameters. Exp Brain Res. 2003;150:127–141. doi: 10.1007/s00221-003-1416-6. [DOI] [PubMed] [Google Scholar]
- 47.Averbeck BB, Crowe DA, Chafee MV, Georgopoulos AP. Neural activity in prefrontal cortex during copying geometrical shapes II: Decoding shape segments from neural ensembles. Exp Brain Res. 2003;150:142–153. doi: 10.1007/s00221-003-1417-5. [DOI] [PubMed] [Google Scholar]
- 48.Agam Y, Sekuler Interactions between working memory and visual perception: An ERP/EEG study. Neu-roimage. 2007;36:933–942. doi: 10.1016/j.neuroimage.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agam Y, Huang J, Sekuler R. Neural correlates of sequence encoding in visuomotor learning. J Neurophysiol. 2010;10:1418–1424. doi: 10.1152/jn.00662.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haller S, Badoud S, Nguyen D, Garibotto V, Lovblad KO, Burkhard PR. Individual detection of patients with Parkinson disease using support vector machine analysis of diffusion tensor imaging data: Initial results. AJNR Am J Neuroradiol. 2012;33:2123–2128. doi: 10.3174/ajnr.A3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He J, Farias S, Martinez O, Reed B, Mungas D, DeCarli C. Differences in brain volume, hippocampal volume, cerebrovascular risk factors, and apolipoprotein E4 among mild cognitive impairment subtypes. Arch Neurol. 2009;66:1393–1399. doi: 10.1001/archneurol.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Cao M, Zhang J. Structural and functional brain changes in the default mode network in subtypes of amnestic mild cognitive impairment. J Geriat Psychia Neurol. 2014;27:188–198. doi: 10.1177/0891988714524629. [DOI] [PubMed] [Google Scholar]
- 53.Raamana PR, Wen W, Kochan NA, Brodaty H, Perminder SS, Wang L, Beg MF. Novel ThickNet features for the discrimination of amnestic MCI subtypes. Neuroimage Clin. 2014;6:284–295. doi: 10.1016/j.nicl.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Zhang ZJ. Neuropsychological and neuroimaging characteristics of amnestic mild cognitive impairment subtypes: A selective overview. CNS Neurosci Therap. 2015;21:776–783. doi: 10.1111/cns.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front Neurosci. 2013;7:32. doi: 10.3389/fnins.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bettcher BM, Mungas D, Patel N, Elofson J, Dutt S, Wynn M, Watson CL, Stephens M, Walsh CM, Kramer JH. Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia. 2016;85:100–109. doi: 10.1016/j.neuropsychologia.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Libon DJ, Gifford K, Holman T, Jefferson AJ. Paper presented at the 44th Annual meeting of the International Neuropsychological Society. Boston, MA: 2016. Dissociating constructs underlying working memory in mild cognitive impairment: A competitive queuing analysis. [Google Scholar]
- 58.Hubbard EM, Piazza M, Pinel P, Dehaene S. Interactions between number and space in parietal cortex. Nat Rev Neurosci. 2005;6:435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- 59.Hoshi Y, Oda I, Wada Y, Ito Y, Yutaka Y, Oda M. Visuospatial imagery is a fruitful strategy for the digit span backward task: A study with near-infrared optical tomography. Cogn Brain Res. 2000;9:339–342. doi: 10.1016/s0926-6410(00)00006-9. [DOI] [PubMed] [Google Scholar]
- 60.Ciulli S, Citi L, Salvadori E, Valenti R, Poggesi A, Inzitari D, Mascalchi M, Toschi N, Pantoni L, Diciotti S. Prediction of impaired performance in Trail Making Test in MCI patients with small vessel disease using DTI data. IEEE J Biomed Health Inform. 2016;20:1026–1033. doi: 10.1109/JBHI.2016.2537808. [DOI] [PubMed] [Google Scholar]
- 61.Kaplan E. A process approach to neuropsychological assessment. In: Boll T, Bryant BR, editors. Clinical neuropsychology and brain function: Research Measurement, and practice: Master lectures. American Psychological Association; Washington, DC: 1988. [Google Scholar]
- 62.Kaplan E. The process approach to neuropsychological assessment of psychiatric patients. J Neuropsychiatry Clin Neurosci. 1990;2:72–87. doi: 10.1176/jnp.2.1.72. [DOI] [PubMed] [Google Scholar]


