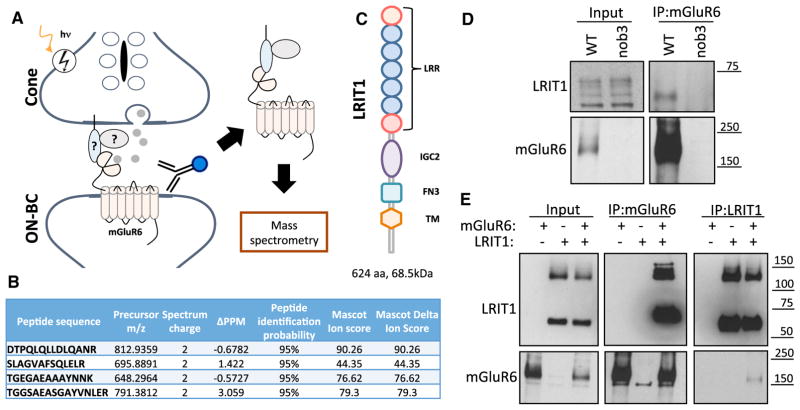
Figure 1. Identification of LRIT1 as mGluR6 Binding Partner.
(A) Schematic of the affinity purification strategy for the identification of the mGluR6 binding partners of mGluR6 at photoreceptor synapses. Specific anti-mGluR6 antibodies were used for the immunoprecipitation from membrane fractions of the retina and the eluates were subjected to mass-spectrometry.
(B) Peptides matching to LRIT1 sequences identified in the mass-spectrometric experiments. Characteristics and parameters used for defining the sequences are shown.
(C) Domain organization of LRIT1. LRR, leucine reach repeat; IGC2, type 2 IgG-like domain; FN3, fibronectin type 3 domain; TM, transmembrane segment.
(D) Verification of mGluR6 interaction with LRIT1 by co-immunoprecipitation from retina lysates. Anti-mGluR6 antibodies were used for the immunoprecipitation (IP) and the presence of LRTI1 and mGluR6 in the IP eluates was detected by western blotting. Retinas lacking mGluR6 (nob3) were used as a specificity control.
(E) Characterization of mGluR6-LRIT1 interaction in transfected HEK293T cells. Both forward and reverse immunoprecipitation experiments using anti-LRIT1 and mGluR6 antibodies were conducted following expression of the indicated constructs and the proteins were detected by western blotting.