Abstract
Patient monitoring on low acuity general hospital wards is currently based largely on intermittent observations and measurements of simple variables, such as blood pressure and temperature, by nursing staff. Often several hours can pass between such measurements and patient deterioration can go unnoticed. Moreover, the integration and interpretation of the information gleaned through these measurements remains highly dependent on clinical judgement. More intensive monitoring, which is commonly used in peri-operative and intensive care settings, is more likely to lead to the early identification of patients who are developing complications than is intermittent monitoring. Early identification can trigger appropriate management, thereby reducing the need for higher acuity care, reducing hospital lengths of stay and admission costs and even, at times, improving survival. However, this degree of monitoring has thus far been considered largely inappropriate for general hospital ward settings due to device costs and the need for staff expertise in data interpretation. In this review, we discuss some developing options to improve patient monitoring and thus detection of deterioration in low acuity general hospital wards.
Introduction
Patients admitted to ICUs, intermediate or high dependency units are usually connected to systems that provide almost continuous monitoring of multiple variables. By contrast, in patients admitted to the general hospital ward (i.e. low acuity settings, such as those defined in the United Kingdom as ‘level 0 or 1 care’ or in the United States as ‘floors’), monitoring is generally limited to intermittent observations and measurements of simple physiological parameters, for example heart rate (HR), respiratory rate and temperature. Yet such patients can be at risk of sudden, unexpected deterioration.
The chain of prevention concept1 has been used to describe the steps required to decrease the likelihood of patient deterioration. The classic model includes staff education, monitoring, recognition of deterioration, how to ‘call for help’ and an effective response. Importantly, each of these components is intimately linked with the others and none on their own will be effective. In this expert opinion review, derived by repeated textual revision among co-authors until consensus was achieved, we will concentrate on how improved monitoring, particularly of respiratory parameters, can help in the recognition of deterioration and why this is important in general ward patients. Aspects of staff education will not be discussed.
Identification of deterioration should be improved
Failure-to-rescue, defined as death of a patient following a complication, is a metric that has been widely used to identify differences in the quality of care between hospitals within healthcare systems.2–4 In a recent prospective international 7-day cohort study of outcomes following elective adult in-patient surgery [International Surgical Outcomes Study (ISOS)],5 44 814 patients were enrolled in 474 hospitals in 19 high, seven middle and one low-income countries; 5270 patients admitted to the hospital ward after surgery (13.2%) developed at least one postoperative complication; 99 of these patients died (1.9%). The ISOS project highlighted not only the impact of patient complications on mortality outcomes but also marked variations among hospitals in failure-to-rescue rates. Importantly, hospitals with the highest complication rates did not have the highest failure-to-rescue rates, suggesting differences in the capability of individual hospitals to identify and escalate the care of patients who develop complications after surgery.6 Variations in failure-to-rescue rates may be related to multiple factors, including patient casemix (differences in demographics, comorbidities, severity of acute illness) but also hospital activity volume, nurse : patient ratios, training of nursing and medical staff, and ability to identify and respond early to patient deterioration.4,7–9
Early identification of deterioration on general hospital wards, enabling rapid targeted management, can help reduce need for transfer to higher acuity units, reduce hospital lengths of stay and costs, and improve survival rates.10,11 Cardoso et al.12 reported that each hour of delay in admission of a patient to the ICU was associated with a 1.5% increase in the risk of death in the ICU and a 1% increase in hospital mortality. Likewise, Sakr et al.13 reported that mortality among critically ill patients was clearly related to the initial evolution of organ failure and the sequential organ failure (SOFA) score at the time of ICU admission. Indeed, more than 50% of all hospitalised patients in that study did not receive optimal treatment before admission to the ICU, and many admissions could have been avoided.14
To detect deterioration sooner, patient monitoring needs to be improved. Indeed, almost 10 years ago, participants at a consensus conference on patient monitoring noted that ‘if practical and affordable, all patents should be monitored continuously’ and identified, in particular, the need to monitor HR, respiratory rate, temperature, pulse oximetry and level of consciousness.15
Improving identification of respiratory deterioration
Respiratory compromise is one of the most common reasons for ICU admission from general hospital wards. Identifying deteriorating respiratory function early could reduce ICU admissions, the need for mechanical ventilation and its associated complications. Several specific groups of patients are at greater risk of respiratory compromise than others. Most obvious are those with chronic respiratory disease. Then there are patients who receive sedation outside the operating room for relatively minor diagnostic or surgical procedures (e.g. dental treatment or endoscopy). Sedation may be accompanied by respiratory depression even some time after the procedure has taken place. In addition, there are patients who receive opioid analgesia, which can be associated with respiratory depression. Lee et al. identified 92 claims for postoperative opioid-induced respiratory depression: 77% of the patients involved had severe brain damage or died. The vast majority of these injuries occurred within 24 h of surgery and 97% were judged to have been preventable with better monitoring and response.16 Clinically significant drug-induced respiratory depression has also been reported with patient-controlled analgesia (PCA).17,18 The Emergency Care Research Institute has recently declared that inadequate monitoring for respiratory depression in patients receiving opioids is one of the top 10 patient safety concerns for healthcare organisations.19
Pulse oximetry
Pulse oximetry is often used to monitor patients in the general hospital ward, because it is noninvasive and provides a rapid indication of oxygenation levels. In a recent study using continuous pulse oximetry in postoperative patients,20 21% of patients were hypoxaemic (SpO2 < 90%) for more than 10 min h−1, 8% averaged more than 20 min hypoxaemia h−1, 37% were hypoxaemic for more than 1 h, 11% for more than 6 h and 3% desaturated below 80% for more than 30 min. Of note, these findings were not captured by nursing staff, whose observations recorded hypoxaemia in only 5% of patients and missed 90% of hypoxaemic events that lasted more than 1 h. The study has limitations as monitoring equipment tended only to be tolerated by those patients who were unable to mobilise (leading to attrition bias), and the generalisability of the findings may be questioned as the average BMI of patients was close to 30 kg m−2 and 16% had obstructive sleep apnoea. In the context of anaesthesia and critical care, it has been acknowledged that pulse oximetry may be misleading or even detrimental as a means of monitoring respiration.21
Unlike the traditional single-alarm threshold value for the SpO2, which is typically chosen arbitrarily and has not been shown to correlate with outcomes, patterns of oxygen saturation can give important clues to a patient's ventilation status. Rapid desaturation and resaturation with corresponding spikes in HR are typical in patients with obstructive sleep apnoea (Fig. 1a). When treated with opioids and sedatives, these patients are at risk of respiratory failure (Fig. 1b). However, supplemental oxygen given routinely and without indication not only hinders the ability of pulse oximetry to detect hypoventilation in a timely manner but may also ‘wash out’ these patterns, so that they are no longer apparent.
Fig. 1.
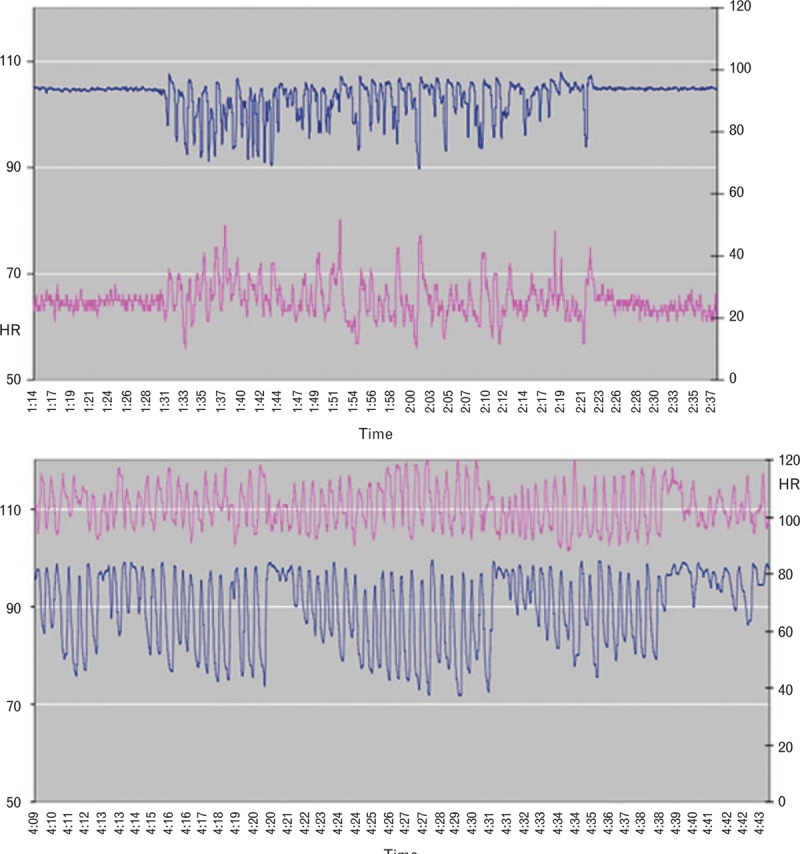
Oxygen saturation (blue) and heart rate (pink) traces in a patient with obstructive sleep apnoea. (a) Preoperatively. (b) Postoperatively during patient-controlled morphine analgesia.
Capnography
An abnormal respiratory rate can be an important indicator of impending complications or deterioration,22,23 but is often not monitored on the general hospital ward, even in patients with known respiratory disease and, when monitored, the methods used are often unreliable. Moreover, it is normally recommended that respiratory rate be counted over a whole minute or two 30-s intervals, and this procedure can represent a significant investment in nursing time in the ward setting, such that accurate rates may only be recorded as little as 37% of the time.24
Capnography, the measurement of CO2 concentrations in respiratory gases, can be performed noninvasively through nasal prongs and offers an accurate and reliable means of measuring respiratory rate, with the availability of instant readings and, when monitored continuously, trends. The respiratory rate is calculated from the frequency of the waveform and changes in the capnography waveform can help identify patient deterioration and the likely cause. For patients already being given additional oxygen, capnography monitoring does not constitute an additional monitoring burden.
There are now clear recommendations for use of continuous capnography in the ICU, cardiac resuscitation and surgical settings,25,26 and studies have demonstrated its effectiveness at identifying respiratory deterioration in general ward patients.27,28 Importantly, capnography provides monitoring of ventilation and, to a certain degree, of pulmonary perfusion and not just monitoring of oxygenation, which is the case with pulse oximetry. Indeed, pulse oximetry may only provide a late alert of respiratory deterioration, particularly if the alarm threshold is set to occur only with sustained desaturation.29 Once oxygen saturation starts to decrease, it decreases quickly, especially in patients at high risk (elderly patients, obese patients, known obstructive sleep apnoea). In addition, patients receiving supplemental oxygen may develop respiratory depression with long periods of apnoea not detected by pulse oximetry.30
Capnography patterns are useful to detect respiratory compromise (Table 1). In Fig. 2, a compressed capnography pattern clearly demonstrates a patient who is experiencing recurrent obstruction of the airway and was observed to be snoring loudly. The pattern when the patient is sleeping soundly but without respiratory compromise is clearly different from that of the patient when they are awake.
Table 1.
Benefits and disadvantages of waveform capnography
| Benefits |
| Detects airflow; it is not a surrogate measure of air flow, such as impedance-based methods that may interpret obstructed chest excursion as ‘breathing’ |
| Can assess adequacy of ventilation |
| Ventilation status remains reliable in patients receiving supplemental oxygen, in whom pulse oximetry detects hypoventilation late |
| Early detection of abnormal respiratory rates or patterns, and of apnoea during acute cardiac or respiratory decompensation |
| Drawbacks |
| Patient compliance is moderate in low acuity settings in which patients are awake and mobile, and especially with ‘scoop’ cannulas, which are required when patients become mouth breathers at deeper levels of sedation |
| Interpretation of ETCO2 waveform requires bedside provider training (although indexes combining parameters simplifies monitoring) |
| False positive low RR, apnoea, and low ETCO2 alarms can be frequent when the cannula is malpositioned |
| Cost of disposables |
| Prone to false alarms for patients on CPAP or BiPAP |
BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; ETCO2, end-tidal CO2; RR, respiratory rate.
Fig. 2.
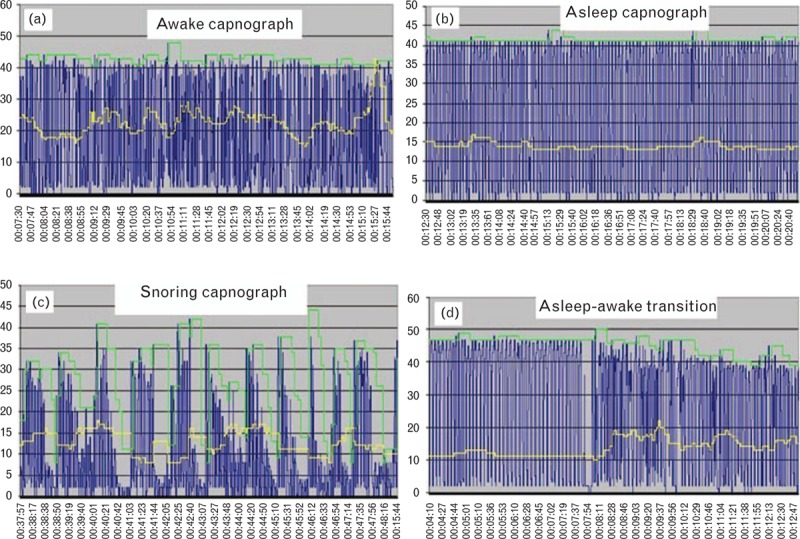
Compressed capnography patterns in a postoperative patient corresponding to different levels of consciousness (yellow line = respiratory rate). Panel (c) shows recurrent partial airway obstruction.
When integrating continuous capnography with oximetry, the expected physiological response to opioid therapy and its potential complications are readily apparent from the tracings. Figure 3 shows a capnography trace from a patient receiving hydromorphone PCA who becomes relatively hypercapnic and bradypnoeic in response to frequent dosing. When the patient is found to be poorly responsive and heavily sedated by the nurse, the pump ‘halt’ feature is enabled and the patient recovers to their baseline, at which time the lockout interval of the PCA pump is increased. Notable is the absence of desaturation on the oximetry trace in this patient due to the administration of supplemental oxygen, reinforcing the value of a monitor of ventilation and frequent level of consciousness assessments.
Fig. 3.
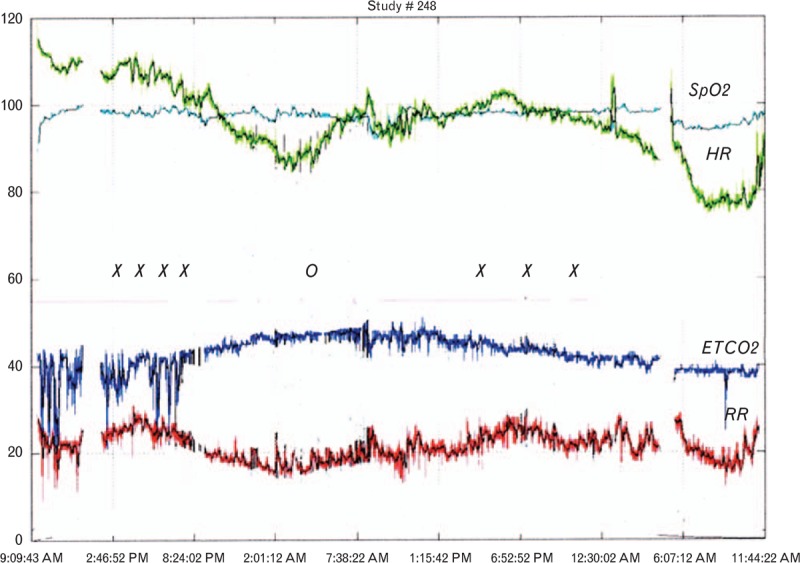
Continuous pulse oximetry and capnography tracings from a postoperative patient receiving hydromorphone patient-controlled analgesia. x = patient-controlled analgesia dosing. o = patient-controlled analgesia halt enabled due to excessive sedation.
Calling for help and response systems
Monitoring systems per se cannot improve outcomes and integral to improved detection of deterioration are correct interpretation of monitored variables to know when to call for help and effective systems to respond to that call.
Early warning scores
Various methods have been developed to identify the patient at risk of deterioration on the general ward. Scoring systems allocate points based on the deviation of a physiological variable from ‘normal’, when measured manually. Some systems trigger a response when individual physiological variables reach a predefined abnormal value. Other more complicated systems allocate points based on the deviation of one or several physiological variables from ‘normal’, and the sum of these points gives a score. This score is then used to determine what response is needed (who to call for help, what to do in the interim until help arrives, what to prepare and when to reassess), often following a predefined hospital-specific or ward-specific escalation protocol. Such scores include the Modified Early Warning Score,31 the National Early Warning Score32 and, more recently, the quick SOFA in patients with suspected sepsis.33
Monitors that integrate several physiological parameters into a single variable indicating patient severity (i.e. automated early warning scores) can also be used rather than systems based on manual measurements. The level of response is then determined by the indicated severity. Such systems are increasingly being developed and tested in traditionally low-monitoring environments,34–36 but further study is needed to assess whether they are associated with improved outcomes.
Early warning systems eliminate the need to rely entirely on the clinical judgement of the nurse for triggering the response and probably also decrease discussion surrounding nurse expectations versus physician response. However, they should not substitute for clinical judgement altogether, nor should they eliminate respect for ‘nurse concern’. Nurses have more direct patient contact than do physicians and should be encouraged to use their intuition when concerned that a patient may be deteriorating. In a systematic review of studies reporting nurse concern, Douw et al.37 noted 170 signs to identify causes of concern and grouped them into 10 categories: change in respiration, change in circulation, rigors, change in mentation, agitation, pain, unexpected trajectory, patient indicating they are feeling unwell, subjective nurse observation and nurse convinced that something is wrong without a rationale. Early warning systems should always leave some option to trigger a response based on nurse concern alone.
Although there does seem to be some evidence suggesting that early warning scores are good predictors of cardiac arrest and death,38 they have not been shown to be associated with improved patient outcome.39 This lack of supportive data is often attributed to the second part of the afferent arm, that is the response to the call for help.
Providing an effective response
An effective response to patient deterioration is best mounted by staff trained and experienced in dealing with acute, critical physiological abnormalities. ‘Rapid Response Teams’ (RRTs) are comprised of healthcare providers who can take intensive care equipment and expertise to patients on the general hospital ward who have early signs of deterioration to prevent further worsening of the condition. RRTs may also be called high acuity response teams or medical emergency teams (METs). The term MET traditionally refers to a specific RRT developed at the Liverpool Hospital in Sydney.
Hospitals began to recognise the potential for RRTs in the early 1990s40 and the concept has expanded such that most hospitals now have some form of RRT in place, encouraged by leading national groups, such as the National Institute for Health and Care Excellence in the United Kingdom41 and the Institute for Healthcare Improvement in the United States.42 Several studies have demonstrated the effectiveness of such teams in reducing the incidence of cardiopulmonary arrests and ICU admissions, and improving patient outcomes.43–47 However, research tying deployment of RRTs to patient outcomes has been hampered by difficulties in measuring processes and outcomes. Most available studies are either observational or have retrospective comparison cohorts, limiting the quality of the results provided. Important components of successful RRTs include accurate criteria for RRT activation, the availability of facilities for patient relocation to an environment with a higher level of monitoring if required, and an administrative and quality improvement component to train staff, collect and analyse event data, provide feed-back, co-ordinate resources and ensure improvement or maintenance over time.
Multiparameter integration and intelligent monitors
Importantly, no single parameter will identify early deterioration in all patients, rather combinations of variables need to be monitored and the information integrated to gain a full picture of patient condition. Vigilance (i.e. the quality of staying alert to the possibility of danger) has been studied very little in medical settings. Signals are more likely to be missed when they occur infrequently.48 More importantly, when the responder is subconsciously aware that they may respond poorly to an alarm or signal, this increases the likelihood that they will miss a rare event.49 Finally, alarms are most likely to be missed when multiple noisy items are present.50 The ideal monitoring system would have 100% sensitivity, that is it would always alarm for a clinically important event, and 100% specificity, that is it would never sound for nonimportant events. Current systems tend to focus on the sensitivity factor, but to achieve this lose specificity so that ‘false’ or ‘nonactionable’ alerts are frequent.51 Many alarms do not need clinical intervention, for example those stemming from sensor malposition or incorrect setting of upper/lower alarm limits.52 False alarms related to sensor displacement due to increased patient mobility are likely to occur more frequently on the general ward than on the much less mobile ICU population. As monitoring increases on the general hospital ward, care needs to be taken to limit the risk of alarm fatigue.53 Physicians and nursing staff rapidly become desensitised to alarm noise and fail to react, adjust the settings to inappropriate values for that patient or simply turn off the alarm completely. It is widely recognised that alarm fatigue can compromise patient safety. Indeed, the 2017 Joint Commission Hospital National Patient Safety Goals include ‘Making improvements to ensure that alarms on medical equipment are heard and responded to on time’.54
There are multiple potential solutions to the challenge of alarm noise, which are beyond the scope of this article. One solution, however, lies in ‘intelligent monitors’ that ‘learn’ to adjust alarms according to trends in the variable being monitored or by cross-checking with other monitored variables.55 Indeed, a large variety of continuous monitoring systems that follow trends and integrate multiple variables to detect patterns pathognomonic of deterioration is now available (Table 2). Integrated monitors may also reduce the numbers of wires and probes needed for each patient and wearable systems that enable patients to be monitored whilst maintaining freedom of movement and mobility are being developed,56,57 particularly important in the general ward patient. Combining monitored values with other patient hospital data (laboratory results, radiology reports, patient comorbid conditions, patient age, risk data) is the next step in developing intelligent monitoring systems; this integration could provide a truly personalised warning system with an alarm activated only when predefined limits enabling identification of deterioration for that specific patient are met (Fig. 4).58–61
Table 2.
Technologies available for continuous monitoring on the general hospital ward
| Device | Vital signs | Technology | Transducer | Sampling location | Connectivity | Ergonomicsa |
| Pulse oximeter | SpO2, HR, RR | Photoplethysmograpy | (1) Transmittance(2) Reflectance | (1) Digit, ear, nasal alae(2) Forehead, chest | (3) Not attached(4) Wireless (Bluetooth, WiFi) | (3) B(4) A− |
| Capnograph | ETCO2, RR | IR spectography | Nasal cannula | Mouth/nose | Attached | C |
| Airflow detector | RR | Humidity detector, thermistor | Face mask, nasal transducer | Mouth/nose | Attached | C |
| Impedance plethysmography | RR, tidal volume | Transthoracic impedance | Electrodes, strain gauges | Chest wall | Attached | B |
| Bioacoustics | RR | Large airway audio (breath) detection | Microphone | Neck | Attached | B |
| Piezoelectric | HR, RR | Piezoelectrics | Piezoelectric element | Under mattress | Hardwired to mattress | A |
| cNIBP | SBP, DBP, MBP | Pulse transit time | Photoplethysmograph, electrodes | Wrist | Wireless | A− |
| Patch (Wearable) | ECG, RR, HR | Accelerometry, electrical impedance | Accelerometer, electrodes | Chest wall | Wireless (Bluetooth, WiFi) | A |
cNIBP, continuous noninvasive blood pressure; ETCO2, end-tidal CO2; HR, heart rate; RR, respiratory rate.
a(A) High patient acceptance due to small transducer not attached to bedside device. (B) Larger transducer (± adhesive) attached to bedside device. (C) Facial transducer often encumbering for awake patients and attached to bedside device.
Fig. 4.
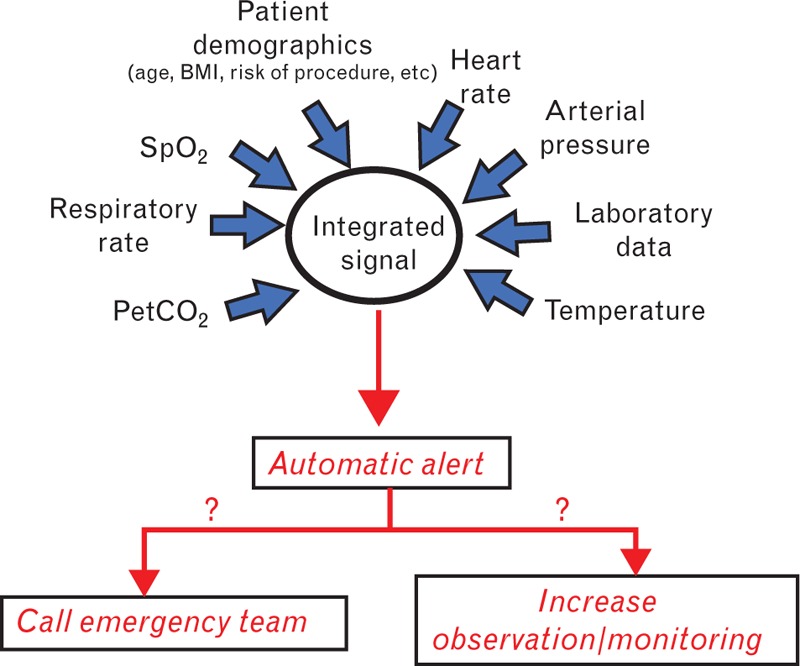
Integrated patient monitoring on the low acuity ward.
Conclusion
Improved monitoring of low acuity ward patients is needed to help reduce failure-to-rescue rates. Contrary to previously accepted perceptions that more complex monitoring is not possible on general wards, there is an increasing body of experience demonstrating that the tools required for such monitoring are not only available but may also be easily used. Clearly the aim of improved monitoring in these areas is not to convert them into ICUs, but to enable early identification of patient deterioration such that an appropriate response can be mounted without increasing nurse workload. Increasingly, monitoring will be automated with devices combining variables to trigger a single alert when combined cut-offs are met. More data are needed to define what these cut-offs should be, which patients will benefit most from more intensive monitoring, and which variables should be monitored in which patients. Importantly, more monitoring, use of an early warning score or availability of an RRT cannot alone reduce failure-to-rescue rates and improve patient outcomes; combined, effective application of all three components is needed and must be adapted to local patient casemix, staff skills and training, and institutional capability.
Acknowledgements relating to this article
Assistance with the study: none.
Financial support and sponsorship: this study was supported by an unrestricted educational grant from Medtronic. The funder had no role or input in writing the report.
Conflicts of interest: SE has received funding for travel, given lectures, owns patents with and/or performed consultancy work for Zoll, Medtronic and Diasorin and has participated in multicentre trials run by Artisanpharma, Eisai and Astra Zeneca. RP holds research grants and has given lectures and/or performed consultancy work for Nestle Health Sciences, BBraun, Medtronic, Glaxo Smithkline and Edwards Lifesciences. SJ has received consulting fees from Drager, Hamilton, Baxter, Xenios and Fisher & Paykel. PK has received lecture fees from FreseniusKabi, MSD, Ratiopharm, Medtronic. FJO is a consultant for Medtronic: Minimally Invasive Therapies Group. DKW has given lectures and/or performed consultancy work for Aguettant and Medtronic. FG has performed consultancy work for Medtronic and received grants for training from Medtronic, Philips, Merck Sharp and Dome and Dräger Medical. AD holds research grants and has given lectures and/or performed consultancy work for MSD, Medtronic, Grünenthal, Medasense, Eurocept. AH has performed consultancy work for BBraun, Edwards, UPmed and Medtronic.
Comment from the editor: PK is an Associate Editor of the European Journal of Anaesthesiology.
Footnotes
Published online 22 February 2018
References
- 1.Smith GB. In-hospital cardiac arrest: is it time for an in-hospital ’chain of prevention’? Resuscitation 2010; 81:1209–1211. [DOI] [PubMed] [Google Scholar]
- 2.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009; 361:1368–1375. [DOI] [PubMed] [Google Scholar]
- 3.Sinha S, Ata OB, Khalid U, et al. Failure-to-rescue and interprovider comparisons after elective abdominal aortic aneurysm repair. Br J Surg 2014; 101:1541–1550. [DOI] [PubMed] [Google Scholar]
- 4.Silber JH, Romano PS, Rosen AK, et al. Failure-to-rescue: comparing definitions to measure quality of care. Med Care 2007; 45:918–925. [DOI] [PubMed] [Google Scholar]
- 5.International Surgical Outcomes Study Group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016; 117:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad T, Bouwman RA, Grigoras I, et al. Use of failure-to-rescue to identify international variation in postoperative care in low-, middle- and high-income countries: a 7-day cohort study of elective surgery. Br J Anaesth 2017; 119:258–266. [DOI] [PubMed] [Google Scholar]
- 7.Aiken LH, Clarke SP, Cheung RB, et al. Educational levels of hospital nurses and surgical patient mortality. JAMA 2003; 290:1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiken LH, Clarke SP, Sloane DM, et al. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA 2002; 288:1987–1993. [DOI] [PubMed] [Google Scholar]
- 9.Ghaferi AA, Dimick JB. Importance of teamwork, communication and culture on failure-to-rescue in the elderly. Br J Surg 2016; 103:e47–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown H, Terrence J, Vasquez P, et al. Continuous monitoring in an inpatient medical-surgical unit: a controlled clinical trial. Am J Med 2014; 127:226–232. [DOI] [PubMed] [Google Scholar]
- 11.Subbe CP, Duller B, Bellomo R. Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care 2017; 21:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardoso LT, Grion CM, Matsuo T, et al. Impact of delayed admission to intensive care units on mortality of critically ill patients: a cohort study. Crit Care 2011; 15:R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakr Y, Lobo SM, Moreno RP, et al. Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care 2012; 16:R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes A, Cecconi M. Can surgical outcomes be prevented by postoperative admission to critical care? Crit Care 2013; 17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeVita MA, Smith GB, Adam SK, et al. Identifying the hospitalised patient in crisis’ – a consensus conference on the afferent limb of rapid response systems. Resuscitation 2010; 81:375–382. [DOI] [PubMed] [Google Scholar]
- 16.Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015; 122:659–665. [DOI] [PubMed] [Google Scholar]
- 17.Overdyk FJ, Carter R, Maddox RR, et al. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg 2007; 105:412–418. [DOI] [PubMed] [Google Scholar]
- 18.Weiniger CF, Carvalho B, Stocki D, et al. Analysis of physiological respiratory variable alarm alerts among laboring women receiving remifentanil. Anesth Analg 2017; 124:1211–1218. [DOI] [PubMed] [Google Scholar]
- 19.ECRI Institute. Top ten patient safety concerns for healthcare organizations. 2017; Available at: https://www.ecri.org/EmailResources/PSRQ/Top10/2017_PSTop10_ExecutiveBrief.pdf. [Accessed 10 February 2018]. [Google Scholar]
- 20.Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg 2015; 121:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McShane AJ, Martin JL. Preoxygenation and pulse oximetry may delay detection of esophageal intubation. J Natl Med Assoc 1987; 79:987.991–987, 992. [PMC free article] [PubMed] [Google Scholar]
- 22.Cretikos MA, Bellomo R, Hillman K, et al. Respiratory rate: the neglected vital sign. Med J Aust 2008; 188:657–659. [DOI] [PubMed] [Google Scholar]
- 23.Fieselmann JF, Hendryx MS, Helms CM, et al. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med 1993; 8:354–360. [DOI] [PubMed] [Google Scholar]
- 24.Benning A, Ghaleb M, Suokas A, et al. Large scale organisational intervention to improve patient safety in four UK hospitals: mixed method evaluation. BMJ 2011; 342:d195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Society of Anesthesiologists. Standards for basic anesthestic monitoring. 2015; Available at: https://www.asahq.org/∼/media/sites/asahq/files/public/resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf. [Accessed 10 February 2018]. [Google Scholar]
- 26.Hinkelbein J, Lamperti M, Akeson J, et al. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol 2017; doi: 10.1097/EJA.0000000000000683. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Stites M, Surprise J, McNiel J, et al. Continuous capnography reduces the incidence of opioid-induced respiratory rescue by hospital rapid resuscitation team. J Patient Saf 2017; doi: 10.1097/PTS.0000000000000408. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.McCarter T, Shaik Z, Scarfo K, et al. Capnography monitoring enhances safety of postoperative patient-controlled analgesia. Am Health Drug Benefits 2008; 1:28–35. [PMC free article] [PubMed] [Google Scholar]
- 29.Rheineck-Leyssius AT, Kalkman CJ. Influence of pulse oximeter lower alarm limit on the incidence of hypoxaemia in the recovery room. Br J Anaesth 1997; 79:460–464. [DOI] [PubMed] [Google Scholar]
- 30.Niesters M, Mahajan RP, Aarts L, et al. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013; 110:837–841. [DOI] [PubMed] [Google Scholar]
- 31.Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified early warning score in medical admissions. QJM 2001; 94:521–526. [DOI] [PubMed] [Google Scholar]
- 32.Royal College of Physicians. National Early Warning Score (NEWS): Standardising the assessment of acute-illness severity in the NHS. Report of a Working Party 2012. Royal College of Physicians, London, 2012. [Google Scholar]
- 33.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones S, Mullally M, Ingleby S, et al. Bedside electronic capture of clinical observations and automated clinical alerts to improve compliance with an early warning score protocol. Crit Care Resusc 2011; 13:83–88. [PubMed] [Google Scholar]
- 35.Tarassenko L, Hann A, Young D. Integrated monitoring and analysis for early warning of patient deterioration. Br J Anaesth 2006; 97:64–68. [DOI] [PubMed] [Google Scholar]
- 36.Ronen M, Weissbrod R, Overdyk FJ, et al. Smart respiratory monitoring: clinical development and validation of the IPI (Integrated Pulmonary Index) algorithm. J Clin Monit Comput 2017; 31:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douw G, Schoonhoven L, Holwerda T, et al. Nurses’ worry or concern and early recognition of deteriorating patients on general wards in acute care hospitals: a systematic review. Crit Care 2015; 19:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith ME, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc 2014; 11:1454–1465. [DOI] [PubMed] [Google Scholar]
- 39.McNeill G, Bryden D. Do either early warning systems or emergency response teams improve hospital patient survival? A systematic review. Resuscitation 2013; 84:1652–1667. [DOI] [PubMed] [Google Scholar]
- 40.Lee A, Bishop G, Hillman KM, et al. The medical emergency team. Anaesth Intensive Care 1995; 23:183–186. [DOI] [PubMed] [Google Scholar]
- 41.National Institute of Clinical Excellence. Acutely ill adults in hospital: recognising and responding to deterioration. 2007; Available at: https://www.nice.org.uk/guidance/cg50. [Accessed 10 February 2018]. [PubMed] [Google Scholar]
- 42.Berwick DM, Calkins DR, McCannon CJ, et al. The 100,000 lives campaign: setting a goal and a deadline for improving healthcare quality. JAMA 2006; 295:324–327. [DOI] [PubMed] [Google Scholar]
- 43.Al-Qahtani S, Al-Dorzi HM, Tamim HM, et al. Impact of an intensivist-led multidisciplinary extended rapid response team on hospital-wide cardiopulmonary arrests and mortality. Crit Care Med 2013; 41:506–517. [DOI] [PubMed] [Google Scholar]
- 44.Ludikhuize J, Brunsveld-Reinders AH, Dijkgraaf MG, et al. Outcomes associated with the nationwide introduction of rapid response systems in the Netherlands. Crit Care Med 2015; 43:2544–2551. [DOI] [PubMed] [Google Scholar]
- 45.Jung B, Daurat A, De JA, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med 2016; 42:494–504. [DOI] [PubMed] [Google Scholar]
- 46.De Jong A, Jung B, Daurat A, et al. Effect of rapid response systems on hospital mortality: a systematic review and meta-analysis. Intensive Care Med 2016; 42:615–617. [DOI] [PubMed] [Google Scholar]
- 47.Maharaj R, Raffaele I, Wendon J. Rapid response systems: a systematic review and meta-analysis. Crit Care 2015; 19:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jerison HJ, Pickett RM. Vigilance: the importance of the elicited observing rate. Science 1964; 143:970–971. [DOI] [PubMed] [Google Scholar]
- 49.Hubal R, Reyes C, Newlin D. Individual differences in vigilance tasks. 2009; Available at: https://sites.duke.edu/ihss/files/2011/12/Hubal_Individ_Diffs_Vigilance_4-23-09_psg.rh_.v2.pdf. [Accessed 10 February 2018]. [Google Scholar]
- 50.Hockney GR. May DN. Effects of noise on human work efficiency. Handbook of noise assessment. New York: Van Nostrand Reinhold; 1978. 335–372. [Google Scholar]
- 51.Chambrin MC, Ravaux P, Calvelo-Aros D, et al. Multicentric study of monitoring alarms in the adult intensive care unit (ICU): a descriptive analysis. Intensive Care Med 1999; 25:1360–1366. [DOI] [PubMed] [Google Scholar]
- 52.Clifford GD, Silva I, Moody B, et al. The PhysioNet/computing in cardiology challenge 2015: reducing false arrhythmia alarms in the ICU. Comput Cardiol 2015; 2015:273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cvach M. Monitor alarm fatigue: an integrative review. Biomed Instrum Technol 2012; 46:268–277. [DOI] [PubMed] [Google Scholar]
- 54.The Joint Commission. 2017 Hospital national patient safety goals. 2017; Available at: https://www.jointcommission.org/assets/1/6/2017_NPSG_HAP_ER.pdf. [Accessed 10 February 2018]. [Google Scholar]
- 55.Michard F. Hemodynamic monitoring in the era of digital health. Ann Intensive Care 2016; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernandez-Silveira M, Ahmed K, Ang SS, et al. Assessment of the feasibility of an ultra-low power, wireless digital patch for the continuous ambulatory monitoring of vital signs. BMJ Open 2015; 5:e006606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darwish A, Hassanien AE. Wearable and implantable wireless sensor network solutions for healthcare monitoring. Sensors (Basel) 2011; 11:5561–5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taenzer AH, Pyke JB, McGrath SP, et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology 2010; 112:282–287. [DOI] [PubMed] [Google Scholar]
- 59.Chopra V, McMahon LF., Jr Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA 2014; 311:1199–1200. [DOI] [PubMed] [Google Scholar]
- 60.Abella Alvarez A, Torrejon Perez I, Enciso Calderon V, et al. ICU without walls project. Effect of the early detection of patients at risk. Med Intensiva 2013; 37:12–18. [DOI] [PubMed] [Google Scholar]
- 61.Huh JW, Lim CM, Koh Y, et al. Activation of a medical emergency team using an electronic medical recording-based screening system. Crit Care Med 2014; 42:801–808. [DOI] [PubMed] [Google Scholar]


