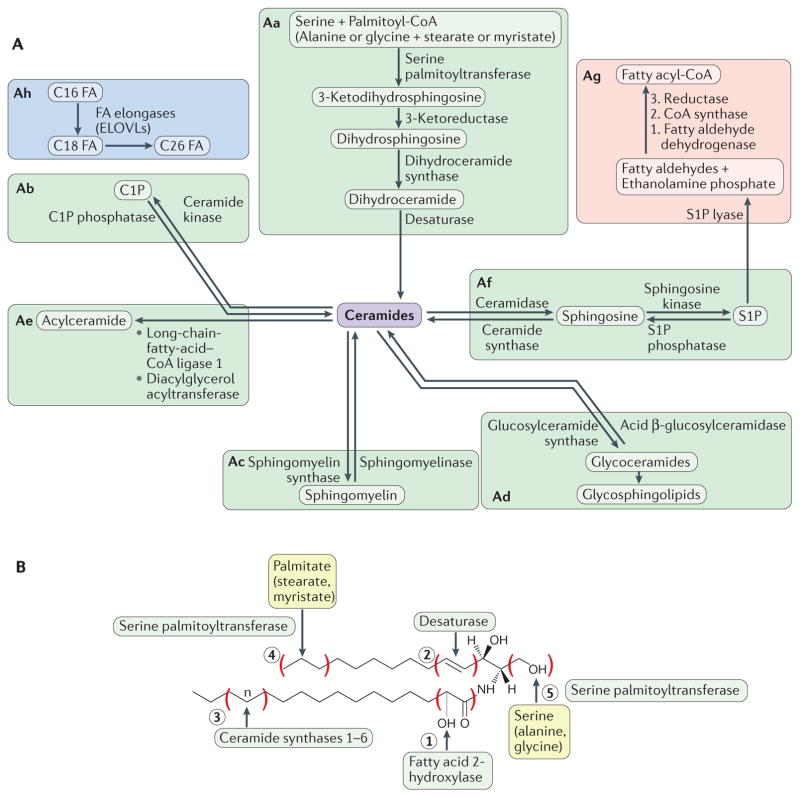
Figure 1. Overview of sphingolipid metabolism.
It is convenient to envision sphingolipid metabolism as organized into blocks. Aa | The de novo biosynthetic pathway is initiated in the endoplasmic reticulum by the action of serine palmitoyltransferase (SPT). Sequential reactions lead to the generation of ceramides. Preferential substrates include serine and palmitoyl-CoA, but alanine or glycine and stearate or myristate can also be used. Ab–Ad | Ceramides are then incorporated into various complex sphingolipids (predominantly in the Golgi) through modifications at the 1-hydroxyl position to generate ceramide-1-phosphate (C1P), sphingomyelin or glycoceramides, which in turn serve as the precursors for the various glycosphingolipids. Ae | Ceramides can be acylated to form 1-O-acyl-ceramides. Af | In sphingolipid catabolic pathways, sphingomyelin, C1P and glycosphingolipids are hydrolysed, resulting in the formation of ceramide (see parts b, c and d). Constitutive catabolism occurs in the lysosome. Ceramide can then be deacylated to generate sphingosine, which in turn is phosphorylated to sphingosine-1-phosphate (S1P). Ag | Exit from sphingolipids is initiated by the action of S1P lyase, which cleaves S1P (or dihydroS1P) to a fatty aldehyde and ethanolamine phosphate. The fatty aldehyde undergoes further metabolism, resulting in the formation of palmitoyl-CoA. Ah | The fatty acid (FA) elongation module is an important adjunct component of the sphingolipid pathway, as sphingolipids are the primary lipids with very-long (C22–26) and ultra-long (longer than C26) fatty acyl chains. B | As the precursor of all complex sphingolipids, ceramides constitute a family of closely related molecules that contain a sphingoid base and an amino-linked FA. Different enzymes introduce variations to the basic structure. Fatty acid 2-hydroxylase introduces an OH on the fatty acyl group (variation 1). Sphingolipidδ (4)-desaturase DES1 (DEGS1) introduces a double bond in the 4–5 position of the sphingoid base (variation 2), whereas DEGS2 can introduce an OH on position 4 (not shown). Ceramide synthases introduce fatty acyl groups of distinct chain length (variation 3). SPT can utilize myristate or stearate instead of palmitate, thus introducing variations in the length of the sphingoid backbone (variation 4). SPT can also utilize alanine or glycine, thus introducing variation 5. For easy nomenclature of ceramides, the chain length of the fatty acyl group is indicated (for example, C18 Cer for the N-stearoyl ceramide). More complete nomenclature indicates the sphingoid base length and degree of FA chain desaturation, for example, 18:1C18 ceramide refers to ceramide with canonical sphingosine and stearate in amide linkage. Non-chemical terminology can also be used, for example, dihydroCer refers to ceramides lacking the 4–5 double bond. ELOVLs, elongation of very-long-chain fatty acids proteins.