Figure 3.
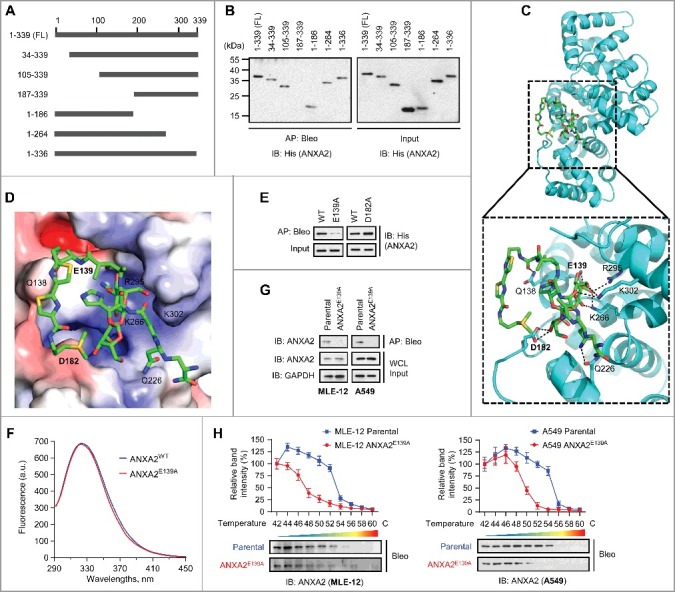
Glu139 of ANXA2 is required for bleomycin binding. (A) Schematic representation of full-length (FL) ANXA2 and its truncation mutants. (B) Recombinant His-ANXA2-FL and its truncation mutants were affinity-purified using bleomycin-immobilized NHS-activated Sepharose beads and then immunoblotted with anti-His antibody. (C and D) Cartoon representation (C) and electrostatic surface representation (D) of the docking model of bleomycin binding to H. sapiens ANXA2. (E) Recombinant His-ANXA2E139A or His-ANXA2D182A was affinity-purified using bleomycin-immobilized NHS-activated Sepharose beads and then immunoblotted with anti-His antibody. (F) Fluorescence quenching assay to examine the conformational changes of recombinant ANXA2 and ANXA2E139A (non-His tagged). (G) Target proteins were affinity-purified with bleomycin-immobilized beads from MLE-12 parental or MLE-12 ANXA2E139A cells and A549 parental or A549 ANXA2E139A cells, and then immunoblotted with anti-ANXA2 antibody. GAPDH, loading control. (H) Cellular thermal shift assay showing ANXA2 or ANXA2E139A target engagement by bleomycin (Bleo) in MLE-12 parental or MLE-12 ANXA2E139A cells (left) and A549 parental or A549 ANXA2E139A cells (right). The thermal stability of ANXA2 was quantified as indicated at the top of each immunoblot. Results are representative of 3 independent experiments unless stated otherwise. Data in (H) are means ± s.d.
