Figure 1.
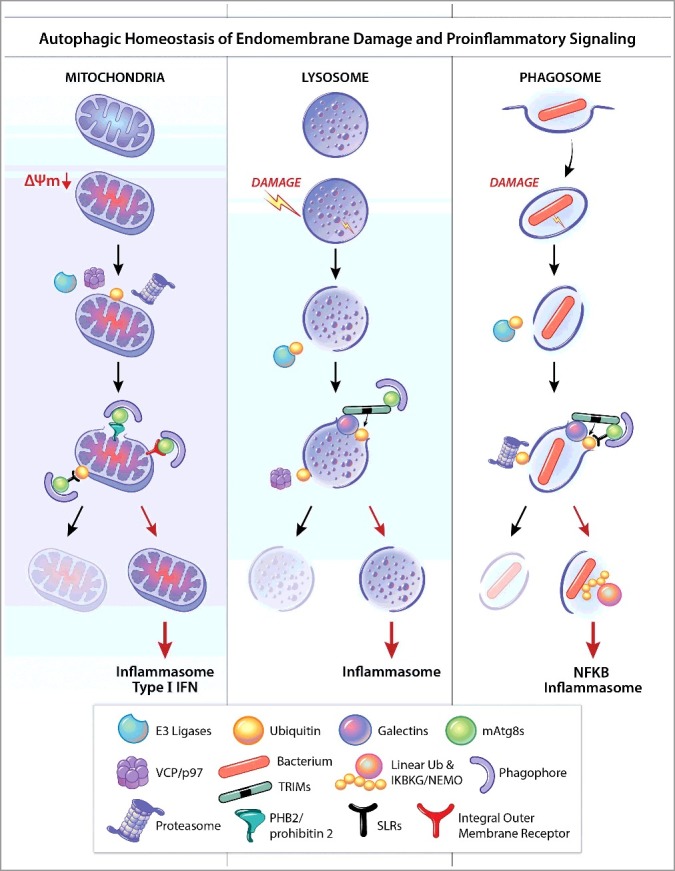
Common themes in autophagic homeostasis of endomembrane damage and proinflammatory signaling. Organelles such as mitochondria (A), lysosomes (B), and phagosomes (C), can be depolarized (ΔΨm), perforated, or otherwise damaged. E3 ligases, such as PRKN/PARK2/parkin, SMURF1, etc., can ubiquitinate targets (K48, K63) on damaged organelles. The AAA-ATPase VCP/p97 can either turn over ubiquitin (K48) by recruiting deubiquitinases to permit further steps in lysosomal homeostasis, or unfold targets such as MFNs (mitofusins) on mitochondria to present them to the proteasome for degradation. K48 ubiquitin also presents phagosomal membrane to proteasomes thus contributing to membrane processing or further damage. Galectins can recognize membrane tears and bind to exposed lumenal glycans while in turn binding to receptors such as SLRs (e.g., LGALS8-CALCOCO2/NDP52), which deliver organelles to phagophores or receptor-regulators (e.g., LGALS3-TRIM16 or LGALS8-TRIM16) that function as receptors and in addition assemble and promote ubiqitination and activation of regulatory ATG factors. SLRs, or other types of receptor such as PHB2 (exposed on the inner membrane of ruptured mitochondria) or integral outer membrane receptors on mitochondria bind Atg8-family proteins (e.g., LC3) via their LC3-interacting region motifs. Damaged organelles are either removed (note crescents representing phagophores, i.e. autophagosome precursors) or otherwise repaired, and if not, they activate inflammasomes (and potentially type I IFNs) as described in the text. If bacteria are not removed along with the remnants of their vacuoles/phagosomes (e.g., via LGALS8), linear ubiquitin chains are formed to activate IKBKG/NEMO and NFKB, leading to inflammation. These processes share common principles and contribute to either suppression of pro-inflammatory responses (by repair or removal of offending organelles and bacteria) or, when they are overwhelmed or otherwise fail, elicit inflammatory cytokines as a second line of defense but at a cost due to associated tissue damage. mATG8s, mammalian Atg8-family proteins.
