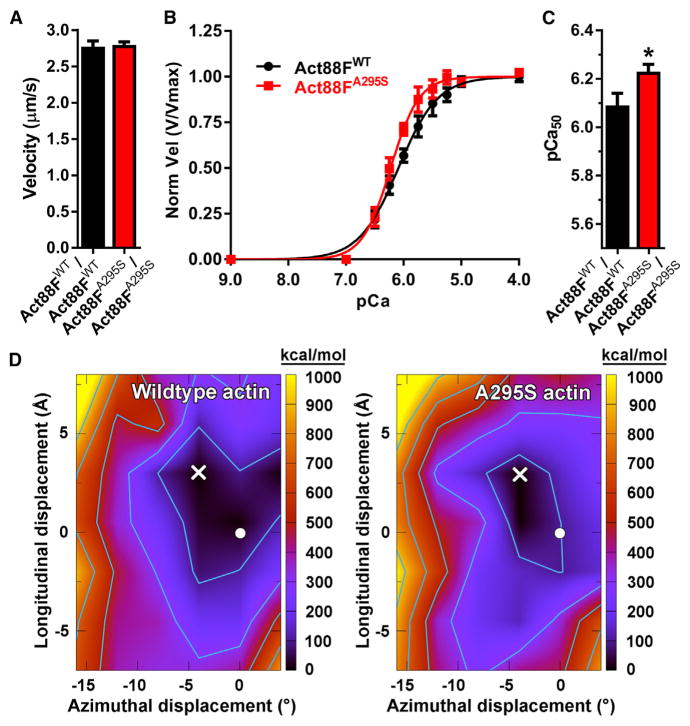
Figure 7. The A295S Actin Mutation Augments Thin Filament Ca2+ Sensitivity and Distorts the F-Actin-Tropomyosin Energy Landscape.
(A) Act88FWT and Act88FA295S F-actin, propelled by rabbit psoas muscle myosin, moved at identical velocities, which suggests the A295S mutation does not markedly affect actomyosin interactions. Average velocities for each of three biological replicate experiments (n = 3) were determined from 20 to 25 filaments per experiment. Bar graphs depict weighted mean velocity ± SEM.
(B) Act88FWT and Act88FA295S-containing thin filaments were reconstituted using bovine cardiac Tn-Tm. Three individual actin preparations were assayed twice (n = 6), and average velocities at each Ca2+ concentration, derived from 20 to 25 filaments per experiment, were pooled, plotted as a function of [Ca2+], and fit to the Hill equation. Act88FWT and Act88FA295S thin filament maximum velocities (Vmax), derived from the fits, were indistinguishable and used to normalize each pooled filament velocity. Normalized velocities ± SEM versus corresponding pCa values are presented.
(C) The pCa50 of reconstituted thin filaments was significantly higher for Act88FA295S relative to Act88FWT filaments, indicative of augmented Ca2+sensitivity. Bar graphs depict pCa50 values ± SEM calculated from the fits. *p = 0.015.
(D) Electrostatic interaction energy landscapes of Tm rotated and translated over the surface of wild-type F-actin (left) and A295S mutant F-actin (right). The color is scaled as indicated on the right axis. Energy values in each graph are relative to the minimum point in the landscape, which was set to zero. The total minimum energy for the wild-type is higher than that for the mutant (−2,165 versus −2,221 kcal/mol, respectively). The minima of the landscapes are denoted with a white X. The position of Tm in the Li et al. (2011) A/B state is indicated by a white circle for reference (Orzechowski et al., 2014). Contours on the plots start at 100 kcal/mol and increase by 200 kcal/mol.
See also Figures S6 and S7 and Tables S3 and S4.