Abstract
AIM
To investigate whether high-mobility group box 1 (HMGB1) Boxb exacerbates BALB/c mice corneal immune responses and inflammatory through the Toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88)-dependent signaling pathway in Aspergillus fumigatus (A. fumigatus) keratitis.
METHODS
The mice corneas were pretreated with phosphate buffer saline (PBS), Boxb before A. fumigatus infection. The abdominal cavity extracted macrophages were pretreated with PBS, Boxb, TLR4 inhibitor (CLI-095), Dimethyl sulfoxide (DMSO) separately before A. fumigatus hyphae stimulation. HMGB1 was detected in normal and infected mice corneas and macrophages by real-time reverse transcriptase polymerase chain reaction (RT-PCR), the TLR4, MyD88, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) were detected by Western blot and PCR.
RESULTS
In BALB/c mice corneas, the expressions of TLR4, HMGB1, IL-1β, TNF-α were increased after A. fumigatus infection. While pretreatment with Boxb significantly increased the expressions of TLR4, HMGB1, MyD88, IL-1β, TNF-α compared with PBS control after infection. In BALB/c mice abdominal cavity extracted macrophages, pretreatment with Boxb increased the expressions of TLR4, HMGB1, MyD88, IL-1β, TNF-α, while pretreatment with CLI-095 and Boxb significantly decreased the expressions of TLR4, HMGB1, MyD88, IL-1β, TNF-α.
CONCLUSION
In A. fumigatus keratitis, Boxb play a pro-inflammatory role in corneal anti-fungi immune response through the HMGB1-TLR4-MyD88 signal pathway.
Keywords: high-mobility group box 1, Aspergillus fumigatus keratitis, Toll-like receptor 4
INTRODUCTION
Fungal keratitis is a severe suppurative ocular disease. It is caused by pathogenic fungi and has a gradually increased incidence in China[1]–[3]. Trauma, corticosteroid or antibiotics abuse, extended usage of contact lens are the predisposing factors of fungal keratitis[4]. The innate immunity of cornea play an important role in defense against pathogenic fungi infection. It is regulated by different pattern recognition receptors (PRRs)[5]–[7]. PRRs could recognize the damage-associated molecular patterns (DAMPs).
Toll-like receptors (TLRs) play an indispensable role in the early detection of DAMPs and orientation of adaptive immunity[8]–[9]. Fungal keratitis can stimulate the expression of various endogenous TLR ligands, especially TLR4, in the damaged corneal tissue[10]. The endogenous molecule high-mobility group box 1 (HMGB1) was known to interaction with TLR4, and was regarded as a proinfalmmatory cytokine that mediates local inflammation, endotoxin lethality, and macrophages activation[11]–[13]. More recently, HMGB1 was recognized as DAMP[14]–[15]. HMGB1 is released by activated monocytes and macrophages after exposure to lipopolysaccharide (LPS), interleukin-1β (IL-1β) or tumor necrosis factor-α (TNF-α), and acted back on macrophages and monocytes by stimulating the synthesis of additional proinfalmmatory cytokines[12],[16]. HMGB1 has two homologous DNA-binding motifs, addressed as HMG A and HMG B boxes[17]–[18]. And Boxb is identified as the proinflammatory domain, which is sufficient to recapitulate the cytokine activity of full-length HMGB1[19]–[20]. There is no evidence showed the role of HMGB1 Boxb in Aspergillus fumigatus (A. fumigatus) infected BALB/c mice corneas. So, we explored the role of Boxb in BALB/c mice corneal innate immunity and the signal pathway of HMGB1/TLR4 in A. fumigatus keratitis.
MATERIALS AND METHODS
Corneal Infection
BALB/c mice (8-week-old female) were purchased from the Changzhou Cavens Laboratory Animal Ltd. The left eyes were selected as experimental eyes. Mice were anesthetized by 8% chloral hydrate and magnified 40× using the stereoscopic microscope, and made a diameter 2 mm scratch on the left eyes central corneal epithelium. Covered the corneal surface by A. fumigatus (No.3.0772, China General Microbiological Culture Collection Center), and contact lens were placed. Eyelids were sewed up. Mice corneas were harvested at 1d after infection. The use of animals in Ophthalmic and Vision Research conformed to the ARVO Statement.
Boxb Treatment
Boxb (0.5 µg/5 µL) or control phosphate buffer saline (PBS; 0.5 µg/5 µL) were given subconjunctivally the day before infection.
Macrophages Extraction
Thioglycollate powde (3 g) was suspended in 100 mL dH2O, stired on heating plate to boiling for 1min. Stored at 4°C. Intraperitoneal (i.p.) injected 1 mL thioglycollate medium and 7d after injection, mice were sacrificed by cervical dislocation. The abdominal was swabbed by 75% alcohol and cut at the middle line through the skin. Slowly injected 6 mL harvest solution with syringe into mice abdominal cavity. Gently massaged the abdomen. Drew out as much of the harvest solution as possible.
Cells Culture and Stimulation
Macrophages cells were seeded in Dulbecco's modified eagle medium (DMEM) and 10% fetal bovine serum (FBS) growth medium. Cells suspensions (1×106/mL) were seeded in 12-well and 6-well culture plates, then cultured at 37°C in a humidified atmosphere containing 5% CO2. Macrophages were pretreated with Boxb (1 µg/mL) or control PBS (1 µg/mL), CLI-095 (InvivoGen) (1 µg/mL) or control DMSO (1 µg/mL) for 2h before infection, then stimulated by A. fumigatus. for 12h and 24h.
Real-time Reverse Transcriptase Polymerase Chain Reaction
Total RNA was isolated from corneas and cells by using RNAiso plus reagent (TaKaRa, Dalian, Liaoning Province, China) and quantified by spectrophotometry rapidly. Use total RNA of 2 µg to produce the complementary DNA by reverse transcription and SYBR Green was used for the quantitative polymerase chain reaction (PCR) reactions. The real-time reverse transcriptase PCR (RT-PCR) primer pair sequences are shown in Table 1. The housekeeping gene β-actin was used as an internal control. Used the 2−ΔΔCt method to quantified.
Table 1. Nucleotide sequences of mouse primers for real-time RT-PCR.
| Gene (5′-3′) | GenBank No. | Primer sequence |
| β-actin | M_007393.3 | F: GATTACTGCTCTGGCTCCTAG C |
| R: GACTCATCGTACTCCTGCTTGC | ||
| TNF-α | M_013693.2 | F: ACCCTCACACTCAGATCATCTT |
| R: GGTTGTCTTTGAGATCCATGC | ||
| TLR4 | NM_021297.3 | F: CCTGACACCAGGAAGCTTGAA |
| R: TCTGATCCATGCATTGGTAGGT | ||
| IL-1β | NM_008361.3 | F: CGCAGCAGCACATCAACAAGAGC |
| R: TGTCCTCATCCTGGAAGGTCCACG | ||
| HMGB1 | NM-000071.6 | F: GGCGAGCATCCTGGCTTATC |
| R: GGCTGCTTGTCATCTGCTG |
Western Blotting
Corneas and macrophages were used to detect the expressions of TLR4, HMGB1, TNF-α and IL-1β protein levels. Harvest the corneas 1d after infection with Boxb or PBS pretreatment. Harvest the macrophages 24h after infection with PBS, Boxb and CLI-095 pretreatment. Corneas and macrophages were lysed in RIPA (Solarbio) lysis buffer contain phenylmethylsulfonyl fluoride (PMSF; 100:1). Total protein was separated on 12% acrylamide SDS-PAGE and transferred onto PVDF membrane (Solarbio). Then blocked the membranes at 37°C for 2h, and incubated with antibody to mouse anti-TLR4, anti-myeloid differentiation primary response 88 (MyD88), anti-TNF-α (Proteintech, Wuhan, China), anti-IL-1β (CST, MA, USA) and β-actin (Bioss, Beijing, China) at 4°C. Then incubated with secondary antibodies at 37°C for 1h. The blots were detected with chemiluminescence (ECL; Thermo Fisher Scientific, Waltham, MA, USA).
Statistical Analysis
The data was expressed as the mean±standard deviation (SD) of at least three independent experiments. Data analysis of cells stimulation was performed by One-way ANOVA test using Graphpad Prism 5.0 software. To analysis the difference of clinical score between two groups, the Mann-Whitney U test were tested. Values were considered to be significant at P<0.05.
RESULTS
Boxb Exacerbated Corneal Inflammation in A. fumigatus Keratitis
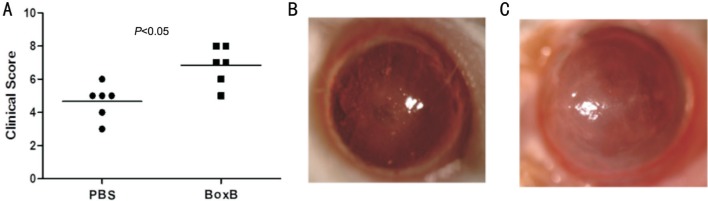
In order to observe the function of Boxb in A. fumigatus keratitis, BALB/c mice models were used to induce A. fumigatus keratitis. The corneas stay normal transparent status after 1d Boxb (0.5 µg/5 µL) or control PBS (0.5 µg/5 µL) were given subconjunctivally into the left eyes of BALB/c mice. But 1d after A. fumigatus infection, significant corneal edema and an irregular whitish mass could be seen (Figure 1B, 1C). And the Boxb group were more severe than the PBS group, as the corneal clinical score indicated in Figure 1A.
Figure 1. Corneal infection by A. fumigatus in BALB/c mice.
A: Clinical scores both increased in corneas infected by A. fumigatus pretreated with PBS and Boxb. Clinical score was shown as mean±SD. PBS (B) and Boxb (C) pretreated corneas were photographed under slit lamp 1d after infection.
Boxb Up-regulated TLR4, HMGB1, IL-1β, TNF-α Expressions
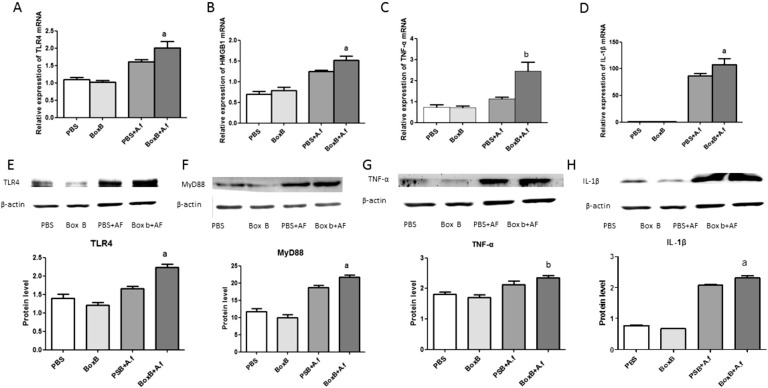
To investigate the function of Boxb in mice corneas after A. fumigatus induced corneal infection, we tested the expressions of TLR4, HMGB1, TNF-α, IL-1β. The real-time RT-PCR results showed that the relative mRNA levels of TLR4, HMGB1, TNF-α, IL-1β were significantly higher in Boxb pretreated mouse corneas than control PBS group at 1d after infection (P<0.05, P<0.05, P<0.01, P<0.05, respectively; Figure 2A-2D). To confirm these data, TLR4, IL-1β and TNF-α protein levels were examined by Western blot. We found that TLR4, IL-1β and TNF-α protein expressions were low in control group, but increased significantly after A. fumigatus infection. The Boxb pretreated group can apparently increased the protein expressions of the TLR4, TNF-α and IL-1β (P<0.05, P<0.01, P<0.05, respectively; Figure 2E, 2G and 2H).
Figure 2. Effect of Boxb treatment on TLR4, MyD88, HMGB1, IL-1β and TNF-α in BALB/c mice corneas.
Corneas of BALB/c mice pretreated with Boxb compared with PBS at 1d. The mRNA expressions of TLR4 (A), HMGB1 (B), TNF-α (C), IL-1β (D) were significantly increased. And the protein levels of TLR4 (E), MyD88 (F), TNF-α (G) and IL-1β (H) were also increased. a P<0.05, bP<0.01 vs PBS+A. fumigatus group.
Boxb Exacerbates Inflammation Through the TLR4/MyD88-dependent Signaling Pathway in Macrophages of BALB/c Mice
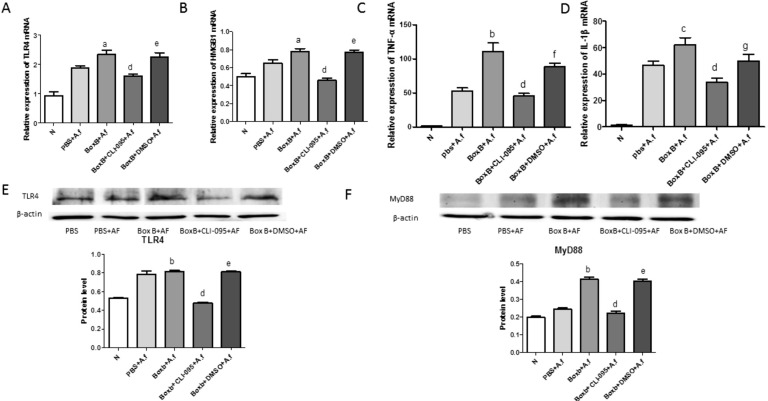
Macrophages are crucial immune cells in A. fumigatus keratitis, and it play a crucial role in the innate immunity. In Figure 2E and 2F, TLR4 and MyD88 both increased in Boxb pretreated corneas. Then in order to explore whether Boxb aggravate fungal keratitis inflammation through the TLR4/MyD88 pathway, CLI-095 and DMSO was added to the abdominal cavity extracted macrophages for 2h, then stimulated with A. fumigatus hyphae and Boxb for 12h. Relative mRNA levels of TLR4, HMGB1, TNF-α and IL-1β were significant higher in Boxb treated group than PBS group (P<0.05, P<0.05, P<0.001, P<0.01, respectively) (Figure 3A-3D). In CLI-095 pretreated group, the relative mRNA levels of TLR4, HMGB1, TNF-α and IL-1β decreased dramatically compared with the Boxb group (P<0.001, P<0.001, P<0.001, P<0.001, respectively) (Figure 3A-3D). TLR4 protein level were tested by Western blot to confirm these data. TLR4 protein levels increased in Boxb group and decreased in CLI-095 group (Figure 3E). And the MyD88 protein level have the same tendency (Figure 3F).
Figure 3. Effects of Boxb treatment on TLR4, MyD88, HMGB1, IL-1β and TNF-α in macrophages of BALB/c mice.
Macrophages treated with Boxb compared with normal, PBS, CLI-095+Boxb, and DMSO+Boxb at 12h. A: The level of TLR4 mRNA was elevated in Boxb+A. fumigatus group, and in the CLI-095+Boxb+A. fumigatus group the TLR4 mRNA was declined; B: The mRNA level of HMGB1 was elevated in Boxb+A. fumigatus group, and the CLI-095 inhibited the HMGB1 mRNA expression; C: The level of TNF-α mRNA was significantly higher in Boxb+A. fumigatus group, and the CLI-095+Boxb+A. fumigatus group the expression was declined; D: The mRNA level of IL-1β was elevated in Boxb+A. fumigatus group, and declined in the CLI-095+Boxb+A. fumigatus group; E: The protein level of TLR4 was elevated in Boxb+A. fumigatus group, and declined in the CLI-095+Boxb+A. fumigatus group; F: The protein level of MyD88 was increased in Boxb+A. fumigatus group, and the CLI-095+Boxb+A. fumigatus group was significantly decreased. a P<0.05; bP<0.001; cP<0.01 vs PBS+A. fumigatus group; dP<0.001 vs Boxb+A. fumigatus group; eP<0.001; fP<0.01; gP<0.05 vs CLI-095+Boxb+A. fumigatus group.
DISCUSSION
HMGB1 was not only acted as a inflammatory responses promoter, but also recognized as a pro-inflammatory cytokine[12]. HMGB1 enlarges and prolongs the inflammatory by mediating cytokine release and regulating anorexia[21], acute lung injury[22], as well as the tissue necrosis[23]. HMGB1 binds to cell surface receptors, such as TLR4, to active the innate immune cells product pro-inflammatory factors, and HMGB1/TLR4 signaling pathway was also found in many inflammatory diseases, like sepsis, rheumatoid arthritis (RA), and arteriosclerosis[24]–[25]. Fungal keratitis is an ulcerative disease of the cornea with severe inflammatory response[26]. But in fungal keratitis, the role of HMGB1 is still not clear. In this study, we investigated the effect of HMGB1 Boxb in A. fumigatus induced corneal infection, and explored its signal pathway.
In severe sepsis and infection caused lethal inflammation syndrome, HMGB1 acted as a necessary mediator. It secreted by innate immune cells, like monocytes, macrophages, neutrophils, and dendritic cells. LPS, TNF-α or IL-1β stimulate the innate immune cells secrete HMGB1, and then stimulate additional pro-inflammation cytokines[12],[16]. In our study, the TNF-α, IL-1β expressions increase both in BALB/c mice fungal keratitis models and its abdominal cavity extracted macrophages. Boxb pretreated mice corneas infected with A. fumigatus have more severe clinical manifestations. The result suggested that fungal keratitis can promote the expression of HMGB1, and the additional HMGB1 may play an proinflammatory role in the progression of A. fumigatus keratitis.
TLRs are important pattern recognition receptors in fungal keratitis[27]. The engagement of TLRs can trigger the innate immune response and induct the adaptive immune response. Interestingly, it has been reported that HMGB1 can stimulate cellular signaling through TLR2, TLR4 and TLR9[28]–[29]. Based on the former studies, we particularly explored the mechanism of how HMGB1 exacerbates fugal keratitis inflammatory through TLR4 signaling pathway. In Boxb subconjunctivally BALB/c mice, the protein levels of TLR4, MyD88, IL-1β and TNF-α increased. And in BALB/c mice abdominal cavity extracted macrophages, when we pretreated with CLI-095, the TLR4 inhibitor, the protein levels of TLR4, MyD88 decreased compared with the Boxb group. So we indicated that in A. fumigatus keratitis, HMGB1 can exacerbates corneal inflammatory through TLR4/MyD88 singling pathway. This finding corroborates the results of those studies HMGB1 binds to TLR4 to trigger proinflammatory cascade.
In conclusion, HMGB1 participates in the innate immune response of A. fumigatus, and acts as a proinflammation cytokine through TLR4/MyD88 signaling pathway.
Acknowledgments
Conflicts of Interest: Liu M, None; Li C, None; Zhao GQ, None; Lin J, None; Che CY, None; Xu Q, None; Xu R, None; Niu YW, None.
REFERENCES
- 1.Li C, Zhao GQ, Che CY, Lin J, Li N, Jia WY, Zhang QQ, Jiang N, Hu LT. Effect of corneal graft diameter on therapeutic penetrating keratoplasty for fungal keratitis. Int J Ophthalmol. 2012;5(6):698–703. doi: 10.3980/j.issn.2222-3959.2012.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen E, Heegaard S, Prause JU, Ivarsen A, Mortensen KL, Hjortdal J. Fungal keratitis - improving diagnostics by confocal microscopy. Case Rep Ophthalmol. 2013;4(3):303–310. doi: 10.1159/000357558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kredics L, Narendran V, Shobana CS, Vágvölgyi C, Manikandan P, Indo-Hungarian Fungal Keratitis Working Group Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses. 2015;58(4):243–260. doi: 10.1111/myc.12306. [DOI] [PubMed] [Google Scholar]
- 4.Xie L, Dong X, Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br J Ophthalmol. 2001;85(9):1070–1074. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marakalala MJ, Kerrigan AM, Brown GD. Dectin-1: a role in antifungal defense and consequences of genetic polymorphisms in humans. Mamm Genome. 2011;22(1-2):55–65. doi: 10.1007/s00335-010-9277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vemuganti GK, Garg P, Gopinathan U, Naduvilath TJ, John RK, Buddi R, Rao GN. Evaluation of agent and host factors in progression of mycotic keratitis: a histologic and microbiologic study of 167 corneal buttons. Ophthalmology. 2002;109(8):1538–1546. doi: 10.1016/s0161-6420(02)01088-6. [DOI] [PubMed] [Google Scholar]
- 7.Fujikawa T, Sakaguchi A, Nishizawa Y, Kouzai Y, Minami E, Yano S, Koga H, Meshi T, Nishimura M. Surface α-1,3-glucan facilitates fungal stealth infection by interfering with innate immunity in plants. PLoS Pathog. 2012;8(8):e1002882. doi: 10.1371/journal.ppat.1002882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 10.Gao X, Zhao G, Li C, Lin J, Jiang N, Wang Q, Hu L, Xu Q, Peng X, He K, Zhu G. LOX-1 and TLR4 affect each other and regulate the generation of ROS in A. fumigatus keratitis. Int Immunopharmacol. 2016;40:392–399. doi: 10.1016/j.intimp.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 12.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 15.Hreggvidsdottir HS, Ostberg T, Wähämaa H, Schierbeck H, Aveberger AC, Klevenvall L, Palmblad K, Ottosson L, Andersson U, Harris HE. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86(3):655–662. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, Tracey KJ. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126(2):389–392. [PubMed] [Google Scholar]
- 17.Weir HM, Kraulis PJ, Hill CS, Raine AR, Laue ED, Thomas JO. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993;12(4):1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardman CH, Broadhurst RW, Raine AR, Grasser KD, Thomas JO, Laue ED. Structure of the A-domain of HMG1 and its interaction with DNA as studied by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry. 1995;34(51):16596–16607. doi: 10.1021/bi00051a007. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Wang H, Warren HS, Moldawer LL, Fink MP, Andersson U, Tracey KJ, Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9(1-2):37–45. [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Wang H, Czura CJ, Tracey KJ. HMGB1 as a cytokine and therapeutic target. J Endotoxin Res. 2002;8(6):469–472. doi: 10.1179/096805102125001091. [DOI] [PubMed] [Google Scholar]
- 21.Agnello D, Wang H, Yang H, Tracey KJ, Ghezzi P. HMGB-1, a DNA-binding protein with cytokine activity, induces brain TNF and IL-6 production, and mediates anorexia and taste aversion. Cytokine. 2002;18(4):231–236. doi: 10.1006/cyto.2002.0890. [DOI] [PubMed] [Google Scholar]
- 22.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 23.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 24.Abe A, Kuwata T, Yamauchi C, Higuchi Y, Ochiai A. High Mobility Group Box1 (HMGB1) released from cancer cells induces the expression of pro-inflammatory cytokines in peritoneal fibroblasts. Pathol Int. 2014;64(6):267–275. doi: 10.1111/pin.12167. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y, Wang H, Mao M, Liang C, Zhang Y, Yang D, Wei Z, Gao S, Hu B, Wang L, Cai Q. Escin increases the survival rate of LPS-induced septic mice through inhibition of HMGB1 release from macrophages. Cell Physiol Biochem. 2015;36:1577–1586. doi: 10.1159/000430320. [DOI] [PubMed] [Google Scholar]
- 26.Jiang N, Zhao G, Lin J, Hu L, Che C, Li C, Wang Q, Xu Q, Peng X. Indoleamine 2,3-Dioxygenase Is Involved in the Inflammation Response of Corneal Epithelial Cells to Aspergillus fumigatus Infections. PLoS One. 2015;10(9):e0137423. doi: 10.1371/journal.pone.0137423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu LT, Du ZD, Zhao GQ, Jiang N, Lin J, Wang Q, Xu Q, Cong L, Qiu S. Role of TREM-1 in response to Aspergillus fumigatus infection in corneal epithelial cells. Int Immunopharmacol. 2014;23(1):288–293. doi: 10.1016/j.intimp.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110(6):1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]