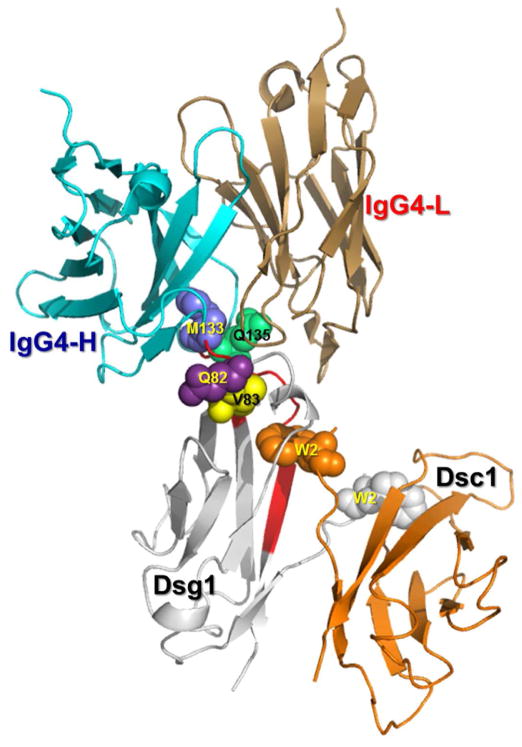
Figure 10. Modeled complex of Fab IgG4 on the modeled Dsg1/Dsc1 heterodimer showing the potential interaction of the FS IgG4 binding region with the Dsg1 conformational epitope.
Residues M133, Q135, Q82 and V83 (blue spheres) of Dsg1 are located close to the hydrophobic acceptor pocket, where the Trp2 (W2) of Dsc1 inserts. The EC1 domains of Dsg1 (magenta) and Dsc1 (pink) are shown as a strand swapped heterodimer, in which both Trp2 residues are exchanged between partnering molecules. It is hypothesized that the binding of FS IgG4 (cyan/sand) to its Dsg1 conformational epitope impairs the heterophilic interaction and adhesion of Dsg1 and Dsc1.