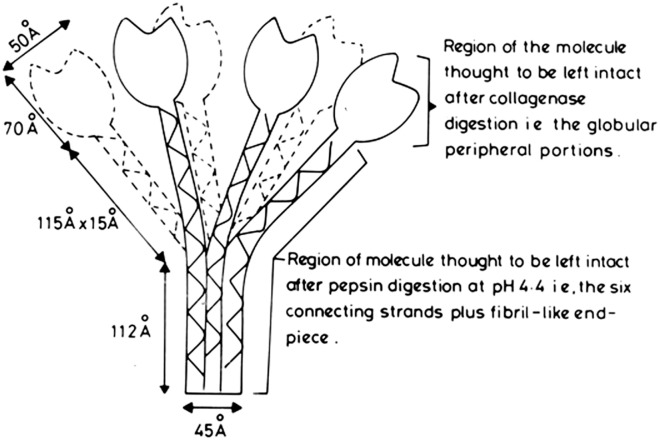
Figure 1.
Proposed model of human subcomponent C1q. Initial diagram, drawn up in 1975, of the first published molecular model proposed for C1q (14). It was based on the electron microscopy measurements (12, 13), the amino acid sequencing and physical chemistry results of the studies on the 190 kDa pepsin-resistant fragment of C1q (1, 11), and the assumption that the collagen-like regions in the A-, B-, and C-chains of C1q form a triple helical collagen-type structure (denoted by the solid, broken, and wavy lines), and the C-terminal approximately 140 amino acid residues, in each of the A-, B-, and C-chains, form a globular heterotrimeric structure of 47.8 kDa (which should, more correctly, be shown as globular units, rather than the tulip-flower shapes as shown in the initial diagram). From the dimensions shown, which are averages of those given in the electron microscopy studies (12), the following comparisons can be made: length of collagen-like fiber plus fibril-like end piece = 115 + 112 = 227 Å. Length of triple helix proposed from amino acid sequences = 78 × 2.9 = 226 Å (2.9 Å is the rise of collagen helix per residue, and there are 78 residues, over the collagen-like regions, in each of the three chains of C1q).