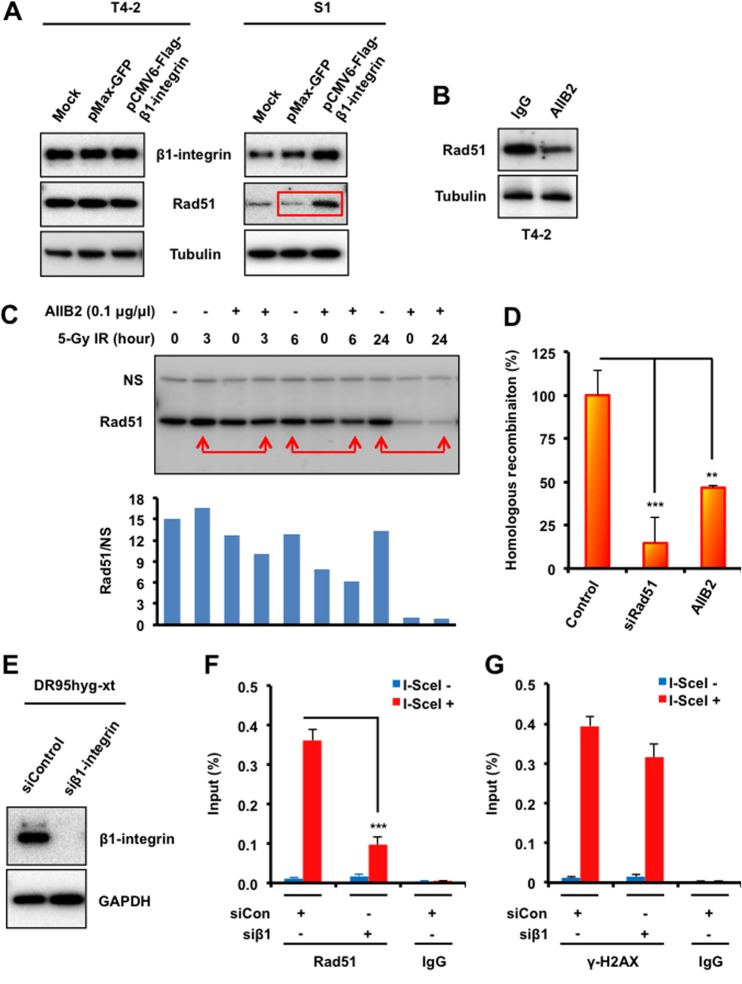
FIG 4.
β1-Integrin regulates Rad51 protein levels and recruitment to DSB sites and promotes HR. (A) T4-2 and S1 cells were electroporated with mammalian expression vectors encoding Flag-tagged β1-integrin or with GFP as a control. Cells were lysed 48 h following electroporation and subjected to immunoblotting with β1-integrin and Rad51 antibodies. Tubulin served as an internal loading control. (B and C) Western blot analysis of Rad51 in whole-cell lysates prepared from malignant breast cancer T4-2 cells treated with the β1-integrin inhibitory monoclonal antibody AIIB2 (0.1 μg/μl) or nonspecific rat IgG before exposure to IR (tubulin served as a loading control). (D) HR was assessed using a DR-GFP reporter assay, as described in Materials and Methods, using T4-2 cells electroporated with control siRNA or Rad51 siRNA or treated with AIIB2 or IgG (“control” represents both control siRNA and IgG). (E) DR95hyg-xt cells were electroporated with either control siRNA or β1-integrin siRNA and harvested after 48 h. Cell lysates were immunoblotted for the indicated proteins. (F and G) ChIP analysis of Rad51 (F) and γ-H2AX (G) on a unique DSB induced by I-SceI in vivo in DR95hyg-xt cells treated with control siRNA (siCon) or β1-integrin siRNA (siβ1), before and after DSB induction by I-SceI transfection. Real-time PCR on ChIP samples used primers directed at nucleotides 94 to 378 from the DSB. The enrichment of Rad51 and γ-H2AX after induction of the DSB was compared with that of an IgG control. (D, F, and G) Columns represent the means (n = 3), and bars represent the SDs; **, P < 0.01; ***, P < 0.001.