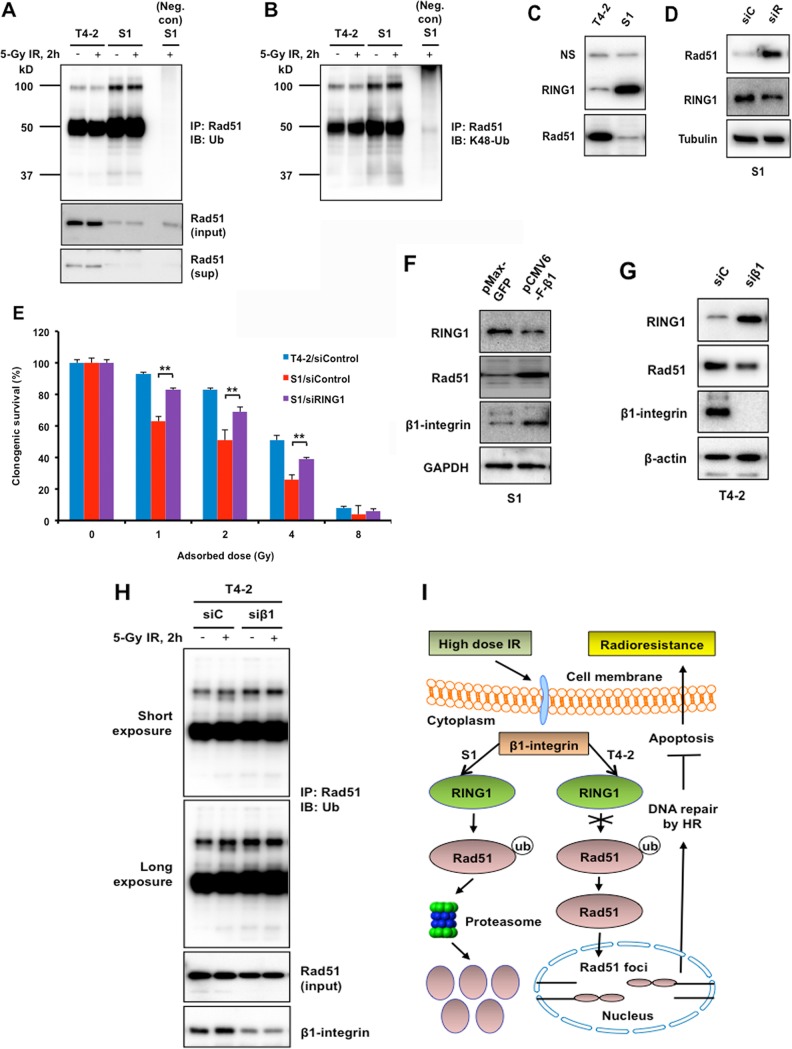
FIG 7.
β1-Integrin regulates Rad51 ubiquitination and Rad51 protein levels by E3 ubiquitin-protein ligase RING1. (A and B) T4-2 and S1 cells were left untreated or treated with 5 Gy of IR. Cells were lysed under denaturing conditions. Rad51 was immunoprecipitated (IP), and immunoblots (IB) were probed with panubiquitin (A) and K48-Ub (B) antibodies. Positions and sizes in kilodaltons of marker proteins are shown on the left. (A) Lower panels show the Rad51 before immunoprecipitation (input) and Rad51 in the supernatant postimmunoprecipitation (sup). Neg. con, negative control. (C and D) Western blot analysis of RING1 and Rad51 expression using whole-cell lysate prepared from T4-2 and S1 cells (C) and from S1 cells electroporated with control siRNA (siC) or Rad51 siRNA (siR) (D). Nonspecific bands (NS) and α-tubulin served as loading controls. (E) Inhibition of RING1 increased radioresistance in S1 cells. Malignant breast T4-2 cells and the nonmalignant S1 breast epithelial cell counterparts were treated with control siRNA or the S1 cells were treated with RING1 siRNA before exposure to 1, 2, 4, or 8 Gy X rays. Clonogenic survival was measured 14 days post-IR. Colonies consisting of more than 50 cells were scored as surviving colonies and normalized against nonirradiated clones (n = 3, mean ± SD; **, P < 0.01). (F and G) Western blot analysis of RING1, Rad51, and β1-integrin expression using whole-cell lysate prepared from S1 cells electroporated with mammalian expression vector for β1-integrin or GFP (control) (F) and from T4-2 cells electroporated with control siRNA (siC) or β1-integrin siRNA (siβ1) (G). GAPDH and β-actin served as loading controls. (H) T4-2 cells, electroporated with either control siRNA (siC) or β1-integrin siRNA (siβ1), were left untreated or were treated with 5 Gy of IR. Cells were lysed under denaturing conditions. Rad51 was immunoprecipitated, and blots were probed with panubiquitin antibody. Lower panels show the Rad51 (input) and β1-integrin before immunoprecipitation. (I) Model of a novel strategy to enhance the efficacy of radiotherapy in breast cancer patients.