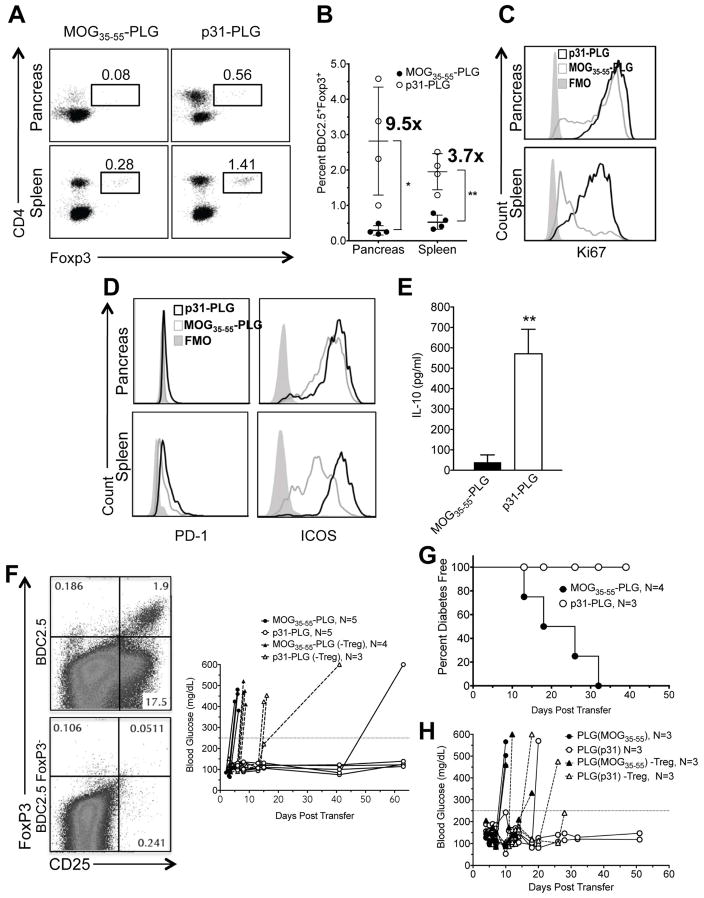
Fig. 6. Ag-PLG treatment induces expansion of Tregs which are required for maintenance of p31-PLG nanoparticle-induced tolerance.
(A–C) Proliferation and expansion of Tregs in p31-PLG treated mice. Diabetes was induced by adoptive transfer of 5×106 p31 activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated 2 hours post transfer with either i.v. MOG35–55-PLG or p31-PLG. Pancreas infiltrating lymphocytes and splenocytes were isolated on day 5 and FACs analysis was used to determine the percentage of CD90.2+CD4+Foxp3+ shown in a representative plot from pooled samples from three mice (A) and numbers or Foxp3+ cells quantitated from 4 individual experiments (B). Ki67 expression on CD90.2+CD4+Foxp3+ BDC2.5 T cells in spleen and pancreas pooled from 3 mice was also determined (C). (D–E) Tregs in p31-PLG treated animals show increased expression of negative costimulatory molecules and production of IL-10 production. Diabetes was induced by adoptive transfer of 5×106 p31-activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated i.v. 2 hours post transfer with either MOG35–55-PLG or p31-PLG. Pancreas infiltrating lymphocytes and splenocytes were isolated on day 5. FACs analysis was used to determine expression of PD-1 and ICOS in p31-PLG (N=3) and MOG35–55-PLG (N=3) treated mice (D). Splenocytes from MOG35–55-PLG (N=3) and p31-PLG (N=3) treated mice were cultured in vitro overnight with p31 peptide and levels of IL-10 secretion in culture supernatants determined by a cytometric bead array (E). (F–H) Tregs are responsible for long-term tolerance maintenance. (F) BDC2.5 CD4+CD25+ T cells were either not depleted or depleted via MACs cell sorting following three days of culture with p31. NOD.SCID mice were infused with activated BDC2.5 cells either containing or not containing CD4+CD25+ T cells and were then treated within two hours of transfer with MOG35–55-PLG or p31-PLG. Individual blood glucose measurements are shown. Results are representative of three separate experiments. (G) Diabetes was induced by adoptive transfer of 5×106 p31-activated BDC2.5 T cells into NOD.SCID recipients. Mice were treated within 2 hours of transfer with either MOG35–55-PLG or p31-PLG nanoparticles. On day 5, splenocytes harvested from both MOG35–55-PLG and p31-PLG treated animals were adoptively transferred into secondary NOD.SCID recipients and blood glucose levels were monitored to determine the incidence of diabetes in the secondary recipient animals. (H) A similar secondary transfer was performed using splenocytes from BDC2.5 NOD.SCID recipients tolerized MOG35–55-PLG or p31-PLG nanoparticles from which CD25+ cells were either not removed or depleted using MACS column sorting. Data from Panels G and H are each representative of two separate experiments.