Abstract
In vitro culture of Plasmodium vivax liver stages underlies key understandings of the fundamental biology of this parasite, particularly the latent, hyponozoite stage, toward drug and vaccine development. Here, we report systematic production of Plasmodium vivax sporozoites in colonized Anopheles darlingi mosquitoes in the Peruvian Amazon. Human subject-derived P. vivax-infected blood was fed to Anopheles darlingi females using standard membrane feedings assays. Optimizing A. darlingi infection and sporozoite production included replacement of infected patient donor serum with naïve donor serum, comparing anticoagulants in processing blood samples, and addition of penicillin–streptomycin and ATP to infectious blood meals. Replacement of donor serum by naïve serum in the P. vivax donor blood increased oocysts in the mosquito midgut, and heparin, as anticoagulant, was associated with the highest sporozoite yields. Maintaining blood-fed mosquitoes on penicillin–streptomycin in sugar significantly extended mosquito survival which enabled greater sporozoite yield. In this study, we have shown that a robust P. vivax sporozoite production is feasible in a malaria-endemic setting where infected subjects and a stable A. darlingi colony are brought together, with optimized laboratory conditions.
Keywords: Plasmodium vivax, Anopheles darlingi, Peruvian Amazon, membrane feeding assays, sporozoite
Malaria remains a global health problem despite enormous, worldwide control efforts. Of the Plasmodium parasites affecting humans, Plasmodium vivax has the largest geographic range with the highest risk in tropical endemic regions. In addition to affecting residents of endemic areas, travelers and military personnel visiting P. vivax-endemic areas are at risk for acute and relapsing malaria due to this parasite. Developing new drug- and vaccine-based primary prevention interventions will rely on better understandings of the biology of P. vivax–hepatocyte interactions. Such work requires access to large numbers of P. vivax sporozoites, which hitherto have been difficult to obtain.
The current inability to study the biology of the hepatic stages of P. vivax, particularly (but not exclusively) hypnozoites, the quiescent liver forms, complicates the goal of malaria elimination. With no current diagnostic method to detect these forms and relapse episodes sustaining malaria transmission, a radical cure seems difficult to achieve.1 In addition, there is a high proportion of blood stage infections that are difficult to detect because asymptomatic carriers and/or submicroscopic infections are undetectable by standard diagnostic methods,2 especially given that most relapses (reactivation of hypnozoites) are asymptomatic.3
One challenge for the study of P. vivax biology is that continuous in vitro culture of P. vivax remains unfeasible,4 making it necessary to use primate models, for example, Plasmodium cynomolgi-infected rhesus macaques,5,6 as source material to approximate P. vivax biology. Although P. vivax hepatocyte development can be studied in in vitro cell culture7 as well as with primary hepatocyte models,8,9 such as the microliver platform,6,10 these are not widely used because P. vivax sporozoites are difficult to obtain. Vaccines based on attenuated sporozoites against Plasmodium falciparum are in different stages of clinical trials,11,12 whereas this type of vaccine has been underexplored for P. vivax, in part due to unavailability of P. vivax sporozoites.
To continuously produce P. vivax sporozoites, an uninterrupted supply of mosquitoes as well as access to infectious gametocytes are required. Anopheles darlingi, the main malaria vector in Amazonia, has been recently established as a stenogamous colony (free-mating).13,14 Susceptibility of A. darlingi to P. vivax infection as well as studies of this vector’s genome and population genetics have been also studied.13,15,16 Several factors have been identified that affect the infection of mosquitoes by malaria parasites. These include environmental factors, such as temperature and humidity,17 genetic factors, such as evasion of mosquito immunity by the parasite,18 and host-related factors such as cytokines.19 The production and development of Plasmodium sporozoites in A. darlingi species has been understudied. Here, we describe strategies and methods (from blood processing to insectary management) that facilitate and improve the production of infective P. vivax sporozoites in a well-established colony of A. darlingi.
Results and Discussion
To validate the vector competence of the Anopheles darlingi colony, paired-infections with the same P. vivax blood donor were performed with lab-reared mosquitoes (from F1 to F12) compared to F1 from wild A. darlingi by SMFA. No significant differences were detected between the control group (F1 from wild A. darlingi females) and mosquitoes from the colony in any experiment regarding infection intensity (geometric mean from the wild 13 (range of 1–93) and in the colony 10 (range of 1–133); Wilcoxon matched-pairs rank test p = 0.3804) or in sporozoite yield per mosquito from the wild (geometric mean 1080 (range of 170–7380) and from the colony 879 (range of 150–6300); Wilcoxon matched-pairs rank test p = 0.3203).
From 2012 to 2016, a total of 219 P. vivax infections in A. darlingi were performed, with a mean age (years) of blood donors of 33.38 (range of 18–55). Parasitemia varied among donors: asexual parasitemia (2 to 112 344 trophozoites per μL, mean 6260 ± 677.2) and gametocytemia (18 to 11 820 gametocytes per μL, mean of 1016 ± 93). In ten experiments, oocyst numbers were not recorded because of logistical issues. Eight infections failed to produce oocysts in the mosquito midgut (8/209), and then, over 96% of the assays infected mosquitoes. About 60 000 female mosquitoes were employed in the SMFAs of which more than 52 000 were dissected. The infection prevalence (batches where at least one mosquito presented oocysts in the midgut) among the positive infections (n = 201) was quite high 76 ± 1.8 (±SE), range of 10–100. The geometric mean number of oocysts observed per mosquito infection varied from 1 to 396. Following,20Plasmodium vivax donors could be identified as high (n = 171) and low (n = 24) transmitters, depending on whether geometric mean of oocyst per midgut was ≥1 and infection prevalence was ≥30%.
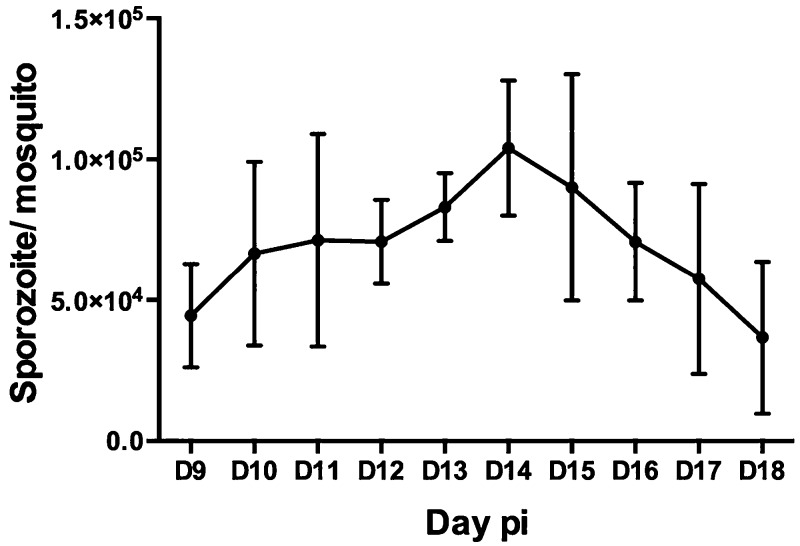
To evaluate when sporozoites appeared in salivary glands, independent time-series dissections (n = 16) (from different P. vivax donors) were performed with at least 20 mosquitoes dissected per infection from day 9 pi to day 18 pi. Despite variability among infections, sporozoite numbers peaked on days 14 and 15 pi (Figure 1), thereafter declining in salivary gland sporozoites.
Figure 1.
Timeline to estimate the number of sporozoites/mosquito after P. vivax infection. Salivary gland dissection started from day 9 pi until day 18 pi (n = 800 mosquitoes). Error bars represent the standard error of the mean of 16 independent experiments.
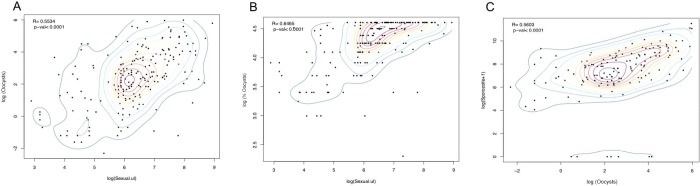
Correlation of P. vivax gametocytemia with density of infection (mean oocysts per mosquito midgut) and prevalence of infection was analyzed with data from 202 SMFAs. Prevalence and intensity of infection in the mosquitoes were significantly correlated with total gametocytemia (R = 0.6465, p < 0.0001; R = 0.5534, p < 0.0001, respectively) (Figures 2A,B and 3).
Figure 2.
Correlation analysis between Plasmodium vivax parasitemia (/μL blood) and (A, B) number of oocysts per midgut and (C) sporozoites in salivary glands in A. darlingi. Spearman’s rank correlation coefficient and bivariate density contours. Total number of infections included in the analysis for oocysts/midgut (n) = 201; total number of infections included for sporozoites analysis (n) = 147.
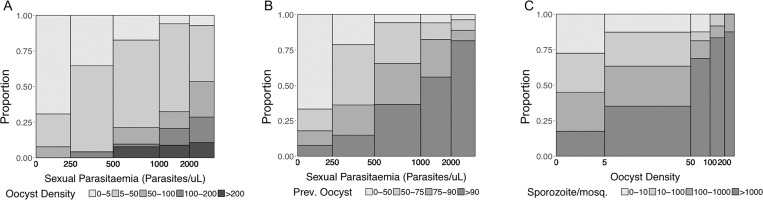
Figure 3.
Proportion of infections according to (A) gametocytemia vs oocyst density, (B) gametocytemia vs infection prevalence, and (C) sporozoite density vs oocyst density. The bar widths are proportional to the number of cases within each category. Total number of infections included in the analysis for oocysts/midgut (n) = 201; total number of infections included for sporozoites analysis (n) = 147.
Salivary gland dissections were performed on 147 SMFA-fed mosquito batches; of these, 7 failed to yield sporozoites. Mean sporozoite yield per mosquito was 6539 (range of 57–98 600), and intensity of infection was correlated with sporozoite production (R = 0.5603; p < 0.0001).
The experiments reported here confirm the susceptibility of free-mating A. darlingi to P. vivax under long-term colony conditions, as evidenced by the successful experimental SMFAs in 201 successful mosquito infections using P. vivax samples collected from 2012 to 2016. Over this time period, more than 157 × 106P. vivax sporozoites were obtained, with variability in the number of sporozoites per mosquito, reaching up to ∼98 000 sporozoites per mosquito. Overall, these data indicate that colonized A. darlingi mosquitoes remain susceptible to P. vivax infection; over 95% of the samples (201/210) produce gametocytes, and 95% (140/147) of the salivary gland dissected contained sporozoites.
The substantial variation in oocyst intensities among assays may be explained by several factors that have been hypothesized to influence transmission, such as gametocyte density,21,22 maturity and sex-ratio of the sexual parasites,23,24 and host–mosquito immune factors, among others. We were not able to identify significant differences in gametocytemia among transmitting and nontransmitting donors. This study was not designed to test any of these other parameters, but future work will focus on how these factors correlate with P. vivax transmission. Overall, there was a correlation between gametocytemia and the number of sporozoites in A. darlingi salivary glands, as other studies in the region have demonstrated.13,22,25
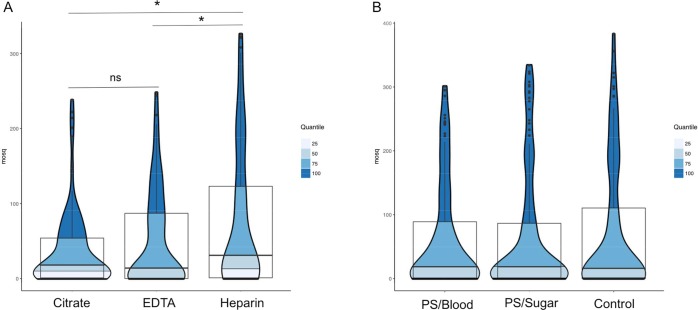
Serum replacement was performed in 14 independent experiments with a significant increase between samples without (geometric mean = 13.51, oocyst range of 1–187) and with (geometric mean = 47.82, oocyst range of 1–324) serum replacement. The effect of EDTA, heparin, and citrate anticoagulants on mosquito infectivity was assessed by experiments with ten different P. vivax donors (blood from the same volunteer was offered to different batches of mosquitoes for each of the treatments) and at least 18 dissected midguts per treatment. A total of 540 mosquitoes were dissected on day 8 pi, and oocysts were enumerated. Using heparin to anticoagulated P. vivax, donor blood was associated with significantly higher oocyst numbers (geometric mean of 74.9; range of 4–322) than use of EDTA (55.30; range of 2–248; nonparametric Mann–Whitney’s test, p = 0.021) or citrate (34.89; range of 4–238; p = 0.016)) (Figure 4A).
Figure 4.
Violin plots showing the distribution of the data where the width of each polygon illustrates the relative frequency of (A) P. vivax oocysts with different anticoagulants in blood donors. EDTA, citrate, and heparin were tested in ten independent assays with a total of 540 dissected mosquitoes. Heparin was significantly different against the other two treatments in the number of oocysts production. (B) P. vivax infectivity oocysts and antibiotic exposure. PS/blood: penicillin and streptomycin mixed with blood; PS/sugar: penicillin and streptomycin mixed with the sugar solution; control: control group. No significant differences were detected.
To assess whether the use of antibacterials affected infection outcomes, 16 experiments using PS were performed in which a total of 600 mosquitoes were fed. Ten mosquitoes for each of the treatments, PS mixed with blood (PS/blood), PS mixed with the sugar solution (PS/sugar) and a control group (without PS), were dissected at day 8 pi (n = 450). Pairwise comparisons of feeding conditions did not indicate a significant increase in the intensity of infection (oocysts/per mosquito midgut) (geometric means of 49.03 (range of 145–301) for PS/sugar, 50.72 (range of 68–321) for PS/blood, and 53.32 (range of 188–322) for the control group (Figure 4B)).
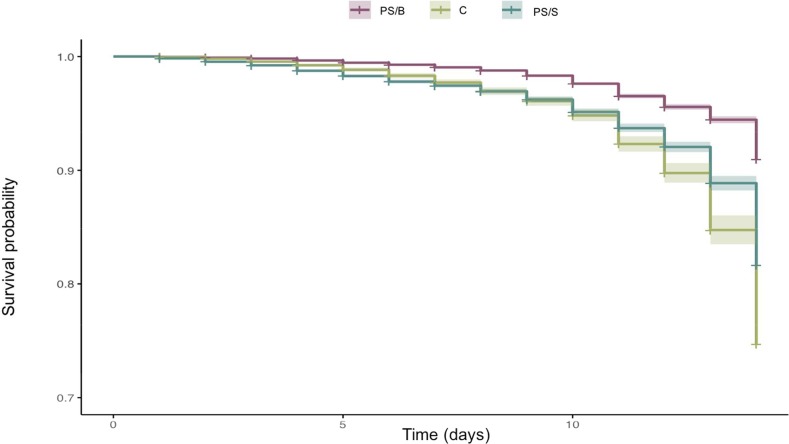
Mosquito survival was assessed in experiments with addition of PS to the routine 10% sugar diet in 15 feeding experiments (n = 7210 mosquitoes), using as a control group mosquitoes feeding only on 10% sugar (n = 5520). Mortality was evaluated in both groups daily from day 1 (after emergence) to day 14. Overall, there was a significant reduction of mortality, from 48% to 26%, when PS was used, and the hazard ratio for death in the PS/sugar treatment was HR = 2.28 (SE ± 0.082; p < 0.0001) and in the control group HR = 2.70 (SE ± 0.114; p < 0.0001) (Figure 5).While addition of PS to sugar maintenance did not have an effect on oocyst production, this treatment enhances survival of fed A. darlingi mosquitoes.
Figure 5.
Kaplan–Meier survival curves of Anopheles darlingi under PS treatments. Mortality was recorded in adult mosquitoes from day 1 to 15. PS/B: Pen/Strep in blood meal; PS/S: Pen/Strep in sugar; C: control, blood meal without Pen/Strep.
The effect of methyl-paraben on mosquito survival was tested in 14 replicates with a total of 450 female mosquitoes in each group. Methyl-paraben had no measurable effect on mosquito survival or on oocyst production.
The inclusion of ATP in the SMFA blood meal to stimulate feeding in A. darlingi was evaluated performing three replicates with a total of 840 female mosquitoes. The proportion of fed mosquitoes was similar to no statistical differences between the concentrations: 86 ± 2 (1 mM), 81 ± 8 (0.1 mM), and 79 ± 2 (0 mM, control). In the presence of exogenous ATP, there was no difference in infection intensity (n = 280): 85.69 (oocyst range of 1–128) (1 mM ATP), 80.01 (oocyst range of 1–144) (0.1 mM ATP), and 78.44 (oocyst range of 1–135) (control, 0 mM ATP).
Different approaches were compared to optimize sporozoite production in the mosquitoes. First, we tested different compounds that are known to influence the parasite development in the mosquito, such as PS or anticoagulants. Second, extending mosquito lifespan increases the number of mosquitoes able to be dissected on days 14 and 15 and, therefore, yields more sporozoites for liver cell infections. Finally, the effect of ATP, a purported mosquito feeding stimulant, was also investigated regarding mosquito feeding intensity, but no effect was seen. Among all the strategies performed, the replacement of serum, use of heparin as anticoagulant to process donor blood, and the inclusion of PS in the sugar solution were associated with improvement in the salivary gland sporozoite yield.
Here, we demonstrate continuous production of Plasmodium vivax sporozoites in the malaria-endemic region of the Peruvian Amazon, where infected subjects and a sustained colony of the major malaria vector Anopheles darlingi are both present. Enhancement of sporozoite yield was affected through various technical measures. Using heparin to collect and process donor blood and penicillin–streptomycin supplementation of maintenance sugar meals during routine insectary maintenance enhanced oocyst and sporozoite yields as well as mosquito survival. The use of heparin is consistent with previously reported results for the development of P. vivax in A. albimanus.(26) Most importantly, we were able to obtain high P. vivax sporozoite yields, sometimes as many as 98 000 sporozoites per mosquito per salivary gland dissection.
Experiments determining P. vivax sporozoite viability and capacity to invade hepatocytes as well as testing antimalarial drugs in hepatic stages are detailed in the accompanying article (Orjuela-Sanchez et al.44). The identification of molecular correlates to detect gametocyte fertility in P. vivax and sporozoite yield would benefit these experiments in terms of reproducibility and robustness.
The data presented here demonstrate a robust and enhanced P. vivax sporozoite yield in the main malaria vector in the Americas, Anopheles darlingi. This work will lead to new drug discovery and vaccine development efforts and to new understandings of the biology and ways to intervene against liver stage parasites, including hypnozoites.1,27
Methods
Plasmodium vivax Donors
To supply infectious gametocytes, donor subjects were enrolled in the city of Iquitos, Department of Loreto, Peru, where P. vivax accounts for ∼80% of malaria cases, with the remaining ∼20% being due to P. falciparum. In 2016, this Department reported ∼54 000 malaria cases,28 representing more than 95% of all malaria cases nationwide. From 2012 to 2016, subjects, ≥18 years old, microscopically diagnosed with P. vivax malaria were invited to participate in the study with written informed consent. All participation was voluntary, and the decision to participate had no effect on treatment, which is provided free of charge by the Peruvian government according to the Peruvian Ministry of Health guidelines. Thick and thin blood smears were prepared from whole blood from each individual volunteer, stained with 10% Giemsa, and examined by experienced microscopists to exclude patient mixed infections with P. falciparum. Parasites and mature gametocyte stage densities were estimated by counting the number of parasites per 500 white blood cells under light microscopy with a 100× immersion oil lens.29 Approximately 10 mL of blood was collected by venipuncture and placed in a constant 37 °C water bath to arrest gametogenesis. These cultures were then used within 1 h of collection for standard membrane blood feeding (SMFA).30
Mosquito Infections
Anopheles darlingi mosquitoes from a laboratory-established colony were laboratory reared as previously described.13 Three-day old A. darlingi female mosquitoes, deprived of sucrose overnight, were placed in small paper cups (number of specimens depended on the experiment) and used for each assay. Between 0.5 and 1 mL of heparinized blood from each volunteer was added to a 1.5 cm diameter glass membrane feeder fitted with a parafilm membrane and maintained at 37 °C during the whole experiment to prevent gametogenesis. Mosquitoes were allowed to feed for 30 min in the dark; then, unfed mosquitoes were removed, and fed mosquitoes were maintained in the insectary at 27 ± 1 °C and 70–80% relative humidity and supplied with sugar water ad libitum.
Oocyst and Sporozoite Counts
Midguts were dissected, stained with 1% mercurochrome, and counted with a phase-contrast microscope (40×) on days 7 and 8 postfeeding to estimate oocyst prevalence (percentage of mosquitoes infected) and infection intensity (number of oocysts/mosquito). It is known that sporozoite density in mosquito salivary glands accumulates over time.31 To determine the optimum time for mosquito dissections to maximize number of P. vivax sporozoites per mosquito, salivary gland dissections of individual specimens were performed, from day 9 postinfection (pi) up to day 18 pi. Mosquitoes were anesthetized at 4 °C, placed in a Petri dish with 70% ethanol, and then washed twice with DMEM sterile medium (Life Technologies #11965118). Salivary glands were dissected under a stereomicroscope, homogenized together (mosquitoes from the same batch) in a glass tissue grinder, filtered through a nylon cell strainer (40 μm pore size), and then centrifuged at 12 000 rpm for 3 min. Sporozoite numbers were counted using a Neubauer chamber hemocytometer using a phase-contrast microscope (40×).
Strategies To Increase Oocysts and Sporozoites Production/Yield in A. darlingi
To identify factors that may improve the yield of infective sporozoites, several experiments were designed.
Serum Replacement of Blood Samples
To assess the effect of plasma on mosquito infection, each blood sample was prepared with and without serum replacement. Plasma was removed from P. vivax-infected packed red cells after centrifugation at 500g for 5 min at 37 °C and replaced with an equal volume of heat-inactivated (30 min at 56 °C) O+ plasma (the most common regional blood type) from donors from Lima, Peru, a nonmalaria endemic area, who reported no travel history to malaria-endemic areas.32 Mosquitoes were separated in two experimental groups (50 mosquitoes per group); one group was offered whole blood and the other, blood with inactivated-blood-serum replacement via MFAs.
Effect of Anticoagulants in P. vivax Oocysts Production
We posited that certain anticoagulants used in blood collection might interfere with parasite viability, exflagellation, and fertilization within the mosquito midgut, hence affecting oocyst production. Several studies have addressed this issue by studying the parasite–mosquito model pairs P. falciparum–A. stephensi33 and P. vivax–A. albimanus.26 To investigate the potential effect on P. vivax development in A. darlingi mosquitoes, three common anticoagulants were tested: EDTA, citrate-phosphate-dextrose, and heparin. Each blood sample was collected and aliquoted into Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) with 6 mL of EDTA, 10 mL of heparin, or 6 mL of citrate and maintained in a 37 °C water bath until membrane feeding to mosquitoes. Then, blood was offered simultaneously to batches of 50 A. darlingi mosquitoes in 16 independent experiments. Midgut dissections were performed on days 7 and 8 postinfection, and mean oocyst counts and prevalence of infection were enumerated using light microscopy.
Effect of Antibiotics on P. vivax Development in A. darlingi
Alterations on mosquito midgut microbiota have been demonstrated to modify parasite infectivity to vectors. Specifically, feeding P. falciparum to A. gambiae in the presence of antibiotics added to sucrose solution increases oocyst counts.34,35 A recent study showed that penicillin and streptomycin (PS) added to donor blood increases mosquito susceptibility to P. falciparum infection as well as enhances mosquito survival rate and fecundity.36 To test the effect of PS on P. vivax oocyst development in A. darlingi, two different strategies were followed: (i) P. vivax-infected blood meal was supplemented with PS (Life Technologies #15070063) and offered to the mosquitoes through SMFA. In this experiment, 20 microliters of a cocktail containing 1000 μg/mL streptomycin plus 1000 U/mL penicillin was added to 1 mL of blood.36 Control blood was supplemented with the same volume of water with added PS. (ii) To supplement the sugar solution where adult mosquitoes were fed with PS (1%), each P. vivax infected blood sample was aliquoted following the above procedures and then was offered to batches of 50 mosquitoes following three combinations: blood + PS and then 10% sugar; blood alone; and then 10% sugar with PS. In addition to oocyst production (midgut dissected on day 8 pi), the effect of PS on mosquito survival was evaluated on mosquitoes fed 10% sugar supplemented with PS, starting at the time of emergence (days 1 to 14).
Use of Methyl-Paraben To Increase Mosquito Survival
Methyl-paraben is an antifungal and antimicrobial routinely used in sugar supplements in mosquito-rearing insectaries. In A. gambiae, the addition of methyl-paraben to the mosquito sugar maintenance meal has been demonstrated to increase mosquitoes survival and infection rates (tested with P. cynomolgi B).37 Here, we assessed the effect of methyl-paraben on the survival rate of A. darlingi and infection with P. vivax. Batches of 25 mosquitoes were fed aliquots from the same blood donor, and then, different treatments were evaluated in three independent experiments (with three replicates per treatment): (i) supplemented postinfection with methyl-paraben (SIGMA #47889) + 10% sugar; (ii) supplemented only with 10% sugar; (iii) 10% sugar + blood feeding.
Effect of ATP on P. vivax Infection of A. darlingi
Adenosine 5′-triphosphate (ATP) has been shown to stimulate feeding in some mosquito species, such as Aedes aegypti, due to the sensitivity of mosquito mouth parts (labral sensilla) to ATP.38,39 To test whether adding ATP to a blood meal increases the mosquito blood intake of blood and amplifies P. vivax burden in mosquitos, blood samples were aliquoted and ATP (SIGMA #A3377) was added at concentrations of 0, 0.1, and 1 mM; the samples were offered to different batches of mosquitoes in three independent experiments (n = 30 mosquitos per batch). The percentage of fed females after SMFA as well as oocysts production was recorded.
Statistical Analysis
Prism 6 (GraphPad Software, San Diego, USA) was used to perform summary statistics of infection prevalence and density and number of sporozoites. Results are presented as geometric means of original values for each batch of mosquitoes. Sporozoite production for each infection was determined from homogenized mosquitoes from independent batches, and then, the yield was adjusted to the number of mosquitoes dissected.40
Statistical analyses were conducted in R v.3.4.3 (R Development Core Ream, R Foundation for Statistical Computing, Australia). Spearman’s rho was calculated to evaluated correlations between continuous skewed data (i.e., oocyst density, oocyst prevalence, gametocytemia, and sporozoite yield) with a significance level of 0.05. For visualization purposes, skewed data were categorized as follows: oocyst density (breaks = 0, 5, 50, 100, 200, >200); oocyst prevalence (0, 50, 75, 90, >90); gametocytemia (0, 250, 500, 750, 1000, 2000, >2000); sporozoite yield (0, 500, 1000, 3000, >3000).41,42
Violin plots with probability quantiles (ggplot2 package) were performed to visualize the density of the data in the use of antibiotic and anticoagulants. Mann-Whitney’s U test was used to assess statistical differences between treatments. The Kaplan-Meier survival curve was used to present survival of mosquitoes exposed to antibiotic vs control group during a 15 day follow-up, and the mortality risk was evaluated with a mixed-effects Cox proportional hazard model (Hazard Ratio: HR).43 The clustering of mosquitoes fed with the same blood donor was modeled as a random effect.
Ethics Statements
This study is part of the Amazonian-ICEMR and was approved by the Ethics Review Board of the Regional Health Direction of Loreto, Universidad Peruana Cayetano Heredia in Lima (R-157-13-14), and the Human Subjects Protection Program of the University of California, San Diego, USA (approval number 120652). Written informed consent was obtained from all study participants prior to subject enrollment and blood sampling.
Acknowledgments
We greatly appreciate the malaria patients for donating their blood samples. We acknowledge Lutecio Torres, Gerson Guedez, Juan Michi, and Christian Rodriguez for their contributions to insectary management and experiments and Zaira Villa for her contributions to sporozoite dissections and counting. We thank Katherine Torres from Universidad Peruana Cayetano Heredia for laboratory assistance and Stephan Meister from UCSD for helping in coordinating activities. We would like to thank Paula Maguiña and Rosa Alban for administrative assistance. This project was funded by Medicines for Malaria Venture (MMV120094/95/96) to E.A.W. and by US National Institutes of Health Cooperative Agreement U19AI089681 and D43TW007120 to J.M.V. B.C. is a full-time employee of MMV. This publication has been possible thanks to the authorization and permits N. 0424-2012-AG-DGFFS-DGEFFS from Direction de Gestión Forestal y de Fauna Silvestre y la Dirección General Forestal y de Fauna Silvestre del Ministerio de Agricultura de la Republica del Peru.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsinfecdis.7b00195.
Supporting Table 1: Database of the Anopheles darlingi feedings on Plasmodium vivax donors in the Peruvian Amazon (PDF)
Author Contributions
M.M., C.T.-R., P.O.-S., G.C.-E., D.G., B.C., E.A.W., and J.M.V. designed the study. M.M. and C.T.-R. performed the experiments. M.M. and G.C.-E. conducted data analysis. M.M. wrote the first draft of the manuscript. M.M., J.M.V., E.A.W., and P.O.-S. modified and finalized the manuscript. All authors read and approved the final manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Olliaro P. L.; Barnwell J. W.; Barry A.; Mendis K.; Mueller I.; Reeder J. C.; Shanks G. D.; Snounou G.; Wongsrichanalai C. (2016) Implications of Plasmodium vivax Biology for Control, Elimination, and Research. Am. J. Trop. Med. Hyg. 95 (6 Suppl), 4–14. 10.4269/ajtmh.16-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Aguirre A.; Gamboa D.; Manrique P.; Conn J. E.; Moreno M.; Lescano A. G.; Sanchez J. F.; Rodriguez H.; Silva H.; Llanos-Cuentas A.; Vinetz J. M. (2016) Epidemiology of Plasmodium vivax Malaria in Peru. Am. J. Trop. Med. Hyg. 95 (6 Suppl), 133–144. 10.4269/ajtmh.16-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede P.; Soto-Calle V. E.; Delgado C.; Gamboa D.; Grande T.; Rodriguez H.; Llanos-Cuentas A.; Anne J.; D’Alessandro U.; Erhart A. (2011) Plasmodium vivax sub-patent infections after radical treatment are common in Peruvian patients: results of a 1-year prospective cohort study. PLoS One 6 (1), e16257. 10.1371/journal.pone.0016257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson-Luque R.; Shaw Saliba K.; Kocken C. H. M.; Pasini E. M. (2017) A Continuous, Long-Term Plasmodium vivax In Vitro Blood-Stage Culture: What Are We Missing?. Trends Parasitol. 33 (12), 921–924. 10.1016/j.pt.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Dembele L.; Franetich J. F.; Lorthiois A.; Gego A.; Zeeman A. M.; Kocken C. H.; Le Grand R.; Dereuddre-Bosquet N.; van Gemert G. J.; Sauerwein R.; Vaillant J. C.; Hannoun L.; Fuchter M. J.; Diagana T. T.; Malmquist N. A.; Scherf A.; Snounou G.; Mazier D. (2014) Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat. Med. 20 (3), 307–312. 10.1038/nm.3461. [DOI] [PubMed] [Google Scholar]
- March S.; Ng S.; Velmurugan S.; Galstian A.; Shan J.; Logan D. J.; Carpenter A. E.; Thomas D.; Sim B. K.; Mota M. M.; Hoffman S. L.; Bhatia S. N. (2013) A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14 (1), 104–115. 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattabongkot J.; Yimamnuaychoke N.; Leelaudomlipi S.; Rasameesoraj M.; Jenwithisuk R.; Coleman R. E.; Udomsangpetch R.; Cui L.; Brewer T. G. (2006) Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am. J. Trop Med. Hyg 74 (5), 708–715. [PubMed] [Google Scholar]
- Mazier D.; Landau I.; Druilhe P.; Miltgen F.; Guguen-Guillouzo C.; Baccam D.; Baxter J.; Chigot J. P.; Gentilini M. (1984) Cultivation of the liver forms of Plasmodium vivax in human hepatocytes. Nature 307 (5949), 367–369. 10.1038/307367a0. [DOI] [PubMed] [Google Scholar]
- Prudencio M.; Mota M. M.; Mendes A. M. (2011) A toolbox to study liver stage malaria. Trends Parasitol. 27 (12), 565–574. 10.1016/j.pt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Khetani S. R.; Bhatia S. N. (2008) Microscale culture of human liver cells for drug development. Nat. Biotechnol. 26 (1), 120–126. 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- Bijker E. M.; Nganou-Makamdop K.; van Gemert G. J.; Zavala F.; Cockburn I.; Sauerwein R. W. (2015) Studying the effect of chloroquine on sporozoite-induced protection and immune responses in Plasmodium berghei malaria. Malar. J. 14, 130. 10.1186/s12936-015-0626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordmuller B.; Surat G.; Lagler H.; Chakravarty S.; Ishizuka A. S.; Lalremruata A.; Gmeiner M.; Campo J. J.; Esen M.; Ruben A. J.; Held J.; Calle C. L.; Mengue J. B.; Gebru T.; Ibanez J.; Sulyok M.; James E. R.; Billingsley P. F.; Natasha K. C.; Manoj A.; Murshedkar T.; Gunasekera A.; Eappen A. G.; Li T.; Stafford R. E.; Li M.; Felgner P. L.; Seder R. A.; Richie T. L.; Sim B. K.; Hoffman S. L.; Kremsner P. G. (2017) Sterile protection against human malaria by chemoattenuated PfSPZ. vaccine. Nature 542 (7642), 445–449. 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M.; Tong C.; Guzman M.; Chuquiyauri R.; Llanos-Cuentas A.; Rodriguez H.; Gamboa D.; Meister S.; Winzeler E. A.; Maguina P.; Conn J. E.; Vinetz J. M. (2014) Infection of laboratory-colonized Anopheles darlingi mosquitoes by Plasmodium vivax. Am. J. Trop. Med. Hyg. 90 (4), 612–616. 10.4269/ajtmh.13-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal-Trevino C.; Vasquez G. M.; Lopez-Sifuentes V. M.; Escobedo-Vargas K.; Huayanay-Repetto A.; Linton Y. M.; Flores-Mendoza C.; Lescano A. G.; Stell F. M. (2015) Establishment of a free-mating, long-standing and highly productive laboratory colony of Anopheles darlingi from the Peruvian Amazon. Malar. J. 14, 227. 10.1186/s12936-015-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart W.; Bickersmith S. A.; Moreno M.; Rios C. T.; Vinetz J. M.; Conn J. E. (2015) Changes in Genetic Diversity from Field to Laboratory During Colonization of Anopheles darlingi Root (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 93 (5), 998–1001. 10.4269/ajtmh.15-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinotti O.; Cerqueira G. C.; de Almeida L. G.; Ferro M. I.; Loreto E. L.; Zaha A.; Teixeira S. M.; Wespiser A. R.; Almeida E. S. A.; Schlindwein A. D.; Pacheco A. C.; Silva A. L.; Graveley B. R.; Walenz B. P.; Lima Bde A.; Ribeiro C. A.; Nunes-Silva C. G.; de Carvalho C. R.; Soares C. M.; de Menezes C. B.; Matiolli C.; Caffrey D.; Araujo D. A.; de Oliveira D. M.; Golenbock D.; Grisard E. C.; Fantinatti-Garboggini F.; de Carvalho F. M.; Barcellos F. G.; Prosdocimi F.; May G.; Azevedo Junior G. M.; Guimaraes G. M.; Goldman G. H.; Padilha I. Q.; Batista Jda S.; Ferro J. A.; Ribeiro J. M.; Fietto J. L.; Dabbas K. M.; Cerdeira L.; Agnez-Lima L. F.; Brocchi M.; de Carvalho M. O.; Teixeira Mde M.; Diniz Maia Mde M.; Goldman M. H.; Cruz Schneider M. P.; Felipe M. S.; Hungria M.; Nicolas M. F.; Pereira M.; Montes M. A.; Cantao M. E.; Vincentz M.; Rafael M. S.; Silverman N.; Stoco P. H.; Souza R. C.; Vicentini R.; Gazzinelli R. T.; Neves Rde O.; Silva R.; Astolfi-Filho S.; Maciel T. E.; Urmenyi T. P.; Tadei W. P.; Camargo E. P.; de Vasconcelos A. T. (2013) The genome of Anopheles darlingi, the main neotropical malaria vector. Nucleic Acids Res. 41 (15), 7387–7400. 10.1093/nar/gkt484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barros F. S.; Honorio N. A.; Arruda M. E. (2011) Survivorship of Anopheles darlingi (Diptera: Culicidae) in relation with malaria incidence in the Brazilian Amazon. PLoS One 6 (8), e22388. 10.1371/journal.pone.0022388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Cruz A.; Canepa G. E.; Kamath N.; Pavlovic N. V.; Mu J.; Ramphul U. N.; Ramirez J. L.; Barillas-Mury C. (2015) Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc. Natl. Acad. Sci. U. S. A. 112 (49), 15178–15183. 10.1073/pnas.1520426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles S. R.; Chuquiyauri R.; Tong C.; Vinetz J. M. (2013) Human host-derived cytokines associated with Plasmodium vivax transmission from acute malaria patients to Anopheles darlingi mosquitoes in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 88 (6), 1130–1137. 10.4269/ajtmh.12-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner G. E.; Ponsa N.; Garman G. W.; Poudel S.; Bell J. A.; Sattabongkot J.; Coleman R. E.; Vaughan J. A. (2006) Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes. Malar. J. 5, 68. 10.1186/1475-2875-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P.; Bousema J. T.; Gouagna L. C.; Otieno S.; van de Vegte-Bolmer M.; Omar S. A.; Sauerwein R. W. (2007) Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am. J. Trop Med. Hyg 76 (3), 470–474. [PubMed] [Google Scholar]
- Rios-Velasquez C. M.; Martins-Campos K. M.; Simoes R. C.; Izzo T.; dos Santos E. V.; Pessoa F. A.; Lima J. B.; Monteiro W. M.; Secundino N. F.; Lacerda M. V.; Tadei W. P.; Pimenta P. F. (2013) Experimental Plasmodium vivax infection of key Anopheles species from the Brazilian Amazon. Malar. J. 12, 460. 10.1186/1475-2875-12-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targett G.; Drakeley C.; Jawara M.; von Seidlein L.; Coleman R.; Deen J.; Pinder M.; Doherty T.; Sutherland C.; Walraven G.; Milligan P. (2001) Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183 (8), 1254–1259. 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- Mitri C.; Thiery I.; Bourgouin C.; Paul R. E. (2009) Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proc. R. Soc. London, Ser. B 276 (1673), 3721–3726. 10.1098/rspb.2009.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti A. R.; Chuquiyauri R.; Brouwer K. C.; Stancil J.; Lin J.; Llanos-Cuentas A.; Vinetz J. M. (2006) Experimental infection of the neotropical malaria vector Anopheles darlingi by human patient-derived Plasmodium vivax in the Peruvian Amazon. Am. J. Trop Med. Hyg 75 (4), 610–616. [PMC free article] [PubMed] [Google Scholar]
- Solarte Y.; Manzano Mdel R.; Rocha L.; Castillo Z.; James M. A.; Herrera S.; Arevalo-Herrera M. (2007) Effects of anticoagulants on Plasmodium vivax oocyst development in Anopheles albimanus mosquitoes. Am. J. Trop Med. Hyg 77 (2), 242–245. [PubMed] [Google Scholar]
- Mueller I.; Galinski M. R.; Baird J. K.; Carlton J. M.; Kochar D. K.; Alonso P. L.; del Portillo H. A. (2009) Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 9 (9), 555–566. 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- http://www.dge.gob.pe/portal/docs/vigilancia/sala/2017/SE02/malaria.pdf.
- WHO . (2010) Basic Malaria Microscopy: Part 1. Learner’s Guide, 2nd ed., WHO, Geneva, Switzerland. [Google Scholar]
- Sinden R. E.; Butcher G. A.; Billker O.; Fleck S. L. (1996) Regulation of infectivity of Plasmodium to the mosquito vector. Adv. Parasitol. 38, 53–117. 10.1016/S0065-308X(08)60033-0. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K.; Jacobs-Lorena M. (2009) Plasmodium sporozoite invasion of the mosquito salivary gland. Curr. Opin. Microbiol. 12 (4), 394–400. 10.1016/j.mib.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattabongkot J.; Tsuboi T.; Hisaeda H.; Tachibana M.; Suwanabun N.; Rungruang T.; Cao Y. M.; Stowers A. W.; Sirichaisinthop J.; Coleman R. E.; Torii M. (2003) Blocking of transmission to mosquitoes by antibody to Plasmodium vivax malaria vaccine candidates Pvs25 and Pvs28 despite antigenic polymorphism in field isolates. Am. J. Trop Med. Hyg 69 (5), 536–541. [PubMed] [Google Scholar]
- Ponnudurai T.; Lensen A. H.; Van Gemert G. J.; Bensink M. P.; Bolmer M.; Meuwissen J. H. (1989) Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98, 165–173. 10.1017/S0031182000062065. [DOI] [PubMed] [Google Scholar]
- Meister S.; Agianian B.; Turlure F.; Relogio A.; Morlais I.; Kafatos F. C.; Christophides G. K. (2009) Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog. 5 (8), e1000542. 10.1371/journal.ppat.1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Dimopoulos G. (2009) Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J. Biol. Chem. 284 (15), 9835–9844. 10.1074/jbc.M807084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrin M.; Rodgers F. H.; Yerbanga R. S.; Ouedraogo J. B.; Basanez M. G.; Cohuet A.; Christophides G. K. (2015) Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat. Commun. 6, 5921. 10.1038/ncomms6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict M. Q.; Hood-Nowotny R. C.; Howell P. I.; Wilkins E. E. (2009) Methylparaben in Anopheles gambiae s.l. sugar meals increases longevity and malaria oocyst abundance but is not a preferred diet. J. Insect Physiol. 55 (3), 197–204. 10.1016/j.jinsphys.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Werner-Reiss U.; Galun R.; Crnjar R.; Liscia A. (1999) Factors modulating the blood feeding behavior and the electrophysiological responses of labral apical chemoreceptors to adenine nucleotides in the mosquito Aedes aegypti (Culicidae). J. Insect Physiol. 45 (9), 801–808. 10.1016/S0022-1910(98)00153-X. [DOI] [PubMed] [Google Scholar]
- Pitts R. J. (2014) A blood-free protein meal supporting oogenesis in the Asian tiger mosquito, Aedes albopictus (Skuse). J. Insect Physiol. 64, 1–6. 10.1016/j.jinsphys.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Swann J.; Corey V.; Scherer C. A.; Kato N.; Comer E.; Maetani M.; Antonova-Koch Y.; Reimer C.; Gagaring K.; Ibanez M.; Plouffe D.; Zeeman A. M.; Kocken C. H.; McNamara C. W.; Schreiber S. L.; Campo B.; Winzeler E. A.; Meister S. (2016) High-Throughput Luciferase-Based Assay for the Discovery of Therapeutics That Prevent Malaria. ACS Infect. Dis. 2 (4), 281–293. 10.1021/acsinfecdis.5b00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T.; Dinglasan R. R.; Morlais I.; Gouagna L. C.; van Warmerdam T.; Awono-Ambene P. H.; Bonnet S.; Diallo M.; Coulibaly M.; Tchuinkam T.; Mulder B.; Targett G.; Drakeley C.; Sutherland C.; Robert V.; Doumbo O.; Toure Y.; Graves P. M.; Roeffen W.; Sauerwein R.; Birkett A.; Locke E.; Morin M.; Wu Y.; Churcher T. S. (2012) Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One 7 (8), e42821. 10.1371/journal.pone.0042821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da D. F.; Churcher T. S.; Yerbanga R. S.; Yameogo B.; Sangare I.; Ouedraogo J. B.; Sinden R. E.; Blagborough A. M.; Cohuet A. (2015) Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Exp. Parasitol. 149, 74–83. 10.1016/j.exppara.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Vaida F.; Xu R. (2000) Proportional hazards model with random effects. Stat Med. 19 (24), 3309–3324. . [DOI] [PubMed] [Google Scholar]
- Orjuela-Sanchez P., Villa Z. H., Moreno M., Tong-Rios C., Meister S., LaMonte G. M., Campo B., Vinetz J. M., and Winzeler E. A.. Developing Plasmodium vivax resources for liver-stage study in the Peruvian Amazon region. ACS Infect. Dis., in press, DOI: 10.1021/acsinfecdis.7b00198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.