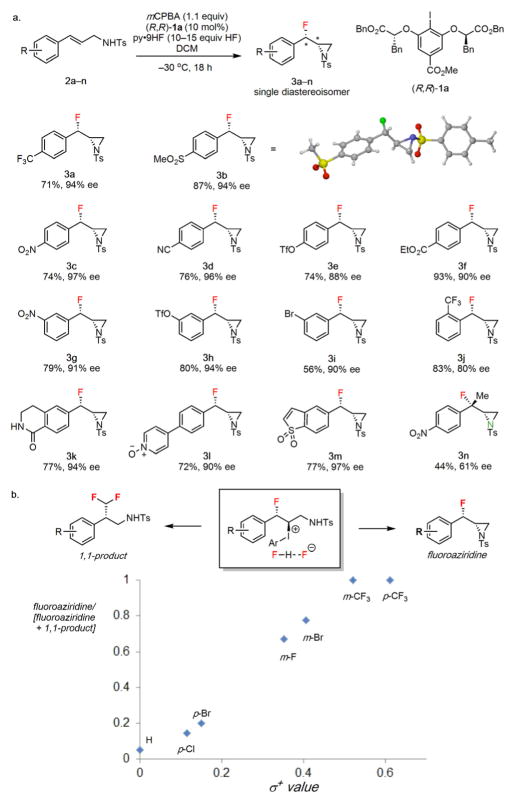
Figure 2.
Evaluation of substrate scope for syn-β-fluoroaziridination. a, Substrate scope evaluation for electron-deficient cinnamyl tosylamides. Isolated yields are indicated below each product (3); conditions: substrate (0.52 mmol), catalyst (10 mol%), mCPBA (0.6 mmol), pyr•9HF (7.5 mmol HF) in DCM (3.0 mL) cooled to –30 °C, 18 h. b, Product selectivity as a function of substituent. The ratio of fluoroaziridine product yield to the sum of fluoroaziridine and 1,1-difluoromethylated product was determined by 19F NMR analysis of the crude mixture.