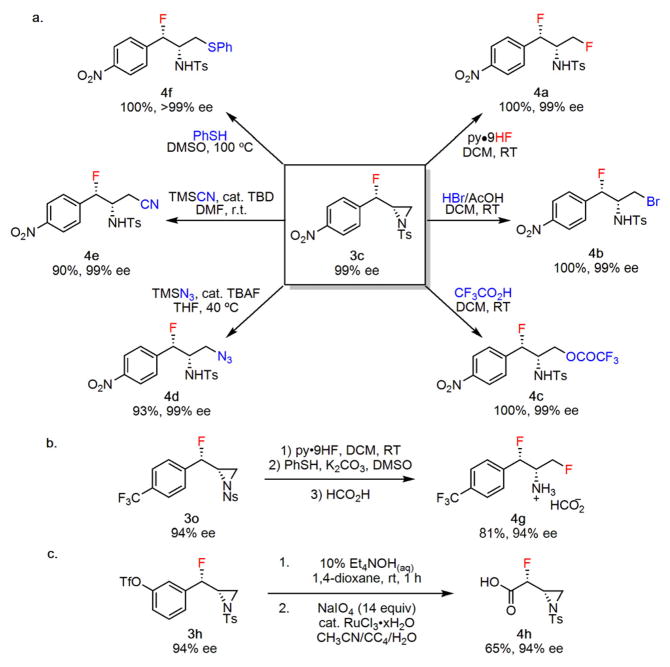
Figure 3.
Synthetic elaboration studies. a, Reactions of fluoroaziri-dine 3c (upgraded from 97 to 99% ee by recrystallization). b, Reactions of the nosyl-protected fluoroaziridine 3o. c, Oxidative degradation of aromatic group in 3h to a carboxylic acid. Isolated yields are indicated below each product (4); experimental details are provided in the Supplementary Information. TBD, triazabicyclodecene; TBAF, tetrabutylammonium fluoride.