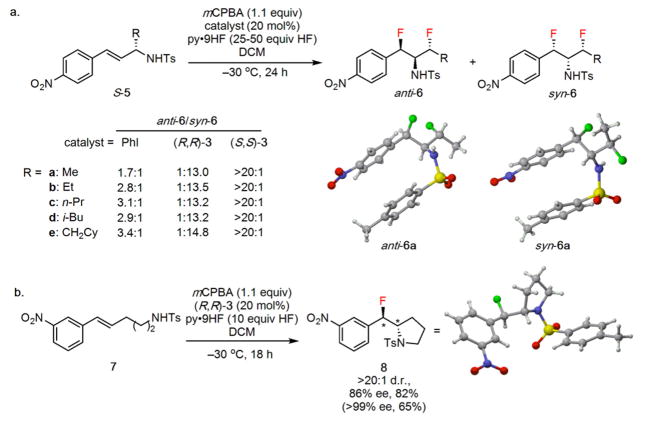
Figure 4.
Extension of the fluoroamination reaction protocol. a, Formation of 1,3-difluoro-2-amines bearing three contiguous ste-reocenters from enantioenriched allylic sulfonamides. b, Fluoroam-ination of bis-homoallylic sulfonamide 7 results in formation of the corresponding anti-β-fluoropyrrolidine. Isolated yields are indicated below all products; the yield in parentheses corresponds to re-crystallized product. Full experimental details are provided in the Supplementary Information.