Abstract
Aim:
Immediate loading protocol, in recent times, has gained popularity as it has not only shortened the treatment time but also resulted in enhanced patient satisfaction. The aim of this study was to evaluate and compare the effectiveness of immediate implant loading protocol over conventional implant loading protocol with respect to peri-implant bone loss.
Materials and Methods:
Twenty patients selected for this study were divided into two groups. In Group I patients, implants were immediately loaded, whereas in Group II, they were loaded with conventional loading protocol. Peri-implant bone loss was measured and compared using intraoral periapical radiographs with the grid at the time of implant loading, 1, 3, and 6 months after implant loading.
Results:
Change in radiographic bone loss in both the groups was found to be statistically significant when baseline was compared to 1, 3, and 6 months, but the difference in the bone loss between Group I and II was not found to be statistically significant.
Conclusion:
No statistically significant difference was observed in the crestal bone loss on comparison of immediate loading to delayed loading protocol.
Clinical Significance:
After achieving good primary stability, immediate-loaded implants can be used for the benefit of the patients as it reduces the period of edentulism.
Keywords: Delayed loading, immediate loading, loading protocols, radiographic levels
INTRODUCTION
Missing teeth have traditionally been replaced with dentures or bridges to restore the ability of patients to eat, speak, and improve appearance.[1] These conventional modalities of rehabilitation have certain limitations such as suboptimal mastication, psychological acceptance, and problems related to esthetics, retention, and stability of the prosthesis. To overcome these, the clinical utilization of dental implants has increased in the recent years.
There are various factors which affect the success rate of implant. Occlusal overload is one such key biomechanical factor which influences implant success as it is the primary factor for generation of peri-implant strain and peri-implant bone loss.[2] Since many patients complained of the discomfort of edentulous spaces during the long healing period of the conventional implant protocol, the concept of immediate loading was proposed by some authors in the early 1990s.[3,4,5] Immediately loaded implants help in counteracting psychological problems as the patient do not have to remain edentulous after placement of implants as in the case of conventionally loaded implants.[3,6] Furthermore, they provide function, comfort, and speech, thus, leading to enhanced patient satisfaction.[3,6]
Trials have reported comparable marginal bone-level changes in immediately and conventionally loaded implants, but the results are contradictory.[7] It has been suggested that immediate loading of the implant may induce micromotion, which could lead to fibrous tissue formation around the implant, and the subsequent implant loss. However, there is no definitive clinical documentation which relates immediate loading to implant failure. Rather it has been reported that low-frequency micromotion may stimulate bone growth.[8,9,10,11] Thus, it would appear that a common factor between immediate loading and delayed loading of dental implants is the initial stability (micromotion) of the implant, implying that close apposition of bone at the time of implant placement may be the fundamental criterion in obtaining osseointegration.[12]
With this background, the present study was undertaken to assess the crestal bone response of different loading protocols for dental implants placed in healed alveolar ridges.
MATERIALS AND METHODS
Patient selection
Twenty partially edentulous patients above 18 years of age reporting to the Department of Prosthodontics of Babu Banarasi Das College of Dental Sciences, Lucknow, desiring replacement of missing teeth were selected for the study, after satisfying the sampling criteria. Criteria of selection included partially edentulous patients who are cooperative, motivated, and committed with completely healed alveolar sockets, have adequate amount of bone volume (buccolingual width not <4 mm and mesiodistal width not <5 mm) and bone quality for implant placement and good periodontal health in the remaining dentition. The exclusion criteria included patients unable/unwilling to undergo minor oral surgical procedure, patients with any known systemic diseases/conditions and/or medication known to interfere with wound healing or minor surgical procedures, smokers, patients with insufficient interarch space to accommodate the required restorative component, patient unable to maintain adequate oral hygiene, and those who are on bisphosphonate therapy or have parafunctional habits.
Division of the patients
Twenty patients were divided randomly into two groups comprising 10 patients in each group as follows (by GraphPad QuickCalcs software)
Test Group I – Immediate loading of the implant after fixture placement, that is, within 48 h
Test Group II – Delayed/conventional loading (CL) of the implant after fixture placement, that is, after 3 months.
All the patients selected had partially edentulous site in the posterior quadrant.
Initial evaluation
Patient preparation included patient education and motivation for optimum oral hygiene regimen. The enrolled patients were subjected to Phase I periodontal therapy (Etiotropic phase). All patients who exhibited good oral hygiene with plaque index and gingival index values of <20% after Phase I therapy were only considered for the study. Patients with periodontal pockets were subjected to pocket elimination or reduction surgeries. Only after a stable periodontal status was attained, patients were selected to be included in the study.
Meticulous evaluation included complete hemograms, casts (study and working model), ridge mapping, photographs, and standardized periapical radiographs with millimeter grid (X-ray mesh). Selection of the diameter and length of the implants were based on study casts, clinical and radiographic evaluation (orthopantomogram) of available bone [Figures 1 and 2]. Surgical stent using self-cure acrylic resin (DPI RR Cold Cure, DPI, Mumbai) was fabricated in all the cases for proper placement of implants. The study protocol was explained to all the patients, and their consent for participating in the study was taken. The individual implant site for all the patients has been presented in Table 1.
Figure 1.
Preoperative cast
Figure 2.
Preoperative orthopantomogram
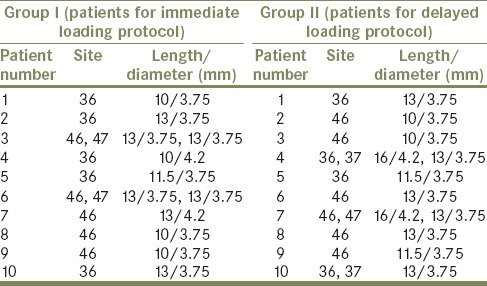
Table 1.
Implant details
Surgical placement of implants
Patients were kept on oral antibiotic a day before implant surgery. After achieving adequate local anesthesia, crestal incisions were placed on the edentulous site with no. 15 blade. The crestal incision was extended to the mid-buccal and mid-lingual crevices of the adjacent tooth. Full-thickness mucoperiosteal flap was elevated using periosteal elevator. The surgical template was inserted and checked for proper positioning. Implant osteotomy site was prepared using a series of drills precisely and incrementally and as per the manufacturer's instructions and site requirement along with profuse irrigation. Bone drilling was performed at revolutionary per minute recommended by Branemark, that is, 1000–1500 rpm [Figure 3]. The depth and angulation were checked continuously with the help of depth gauge, paralleling pins, and by intra-operative radiographs. Once, the depth and angulation of the osteotomy were confirmed, use of subsequent drills for final osteotomy preparation capable of accepting the fixture dimension was accomplished. The implant site was profusely irrigated with sterile saline to ensure no debris or bone chip was left at the base or attached to the vertical walls of the osteotomy site following preparation.
Figure 3.
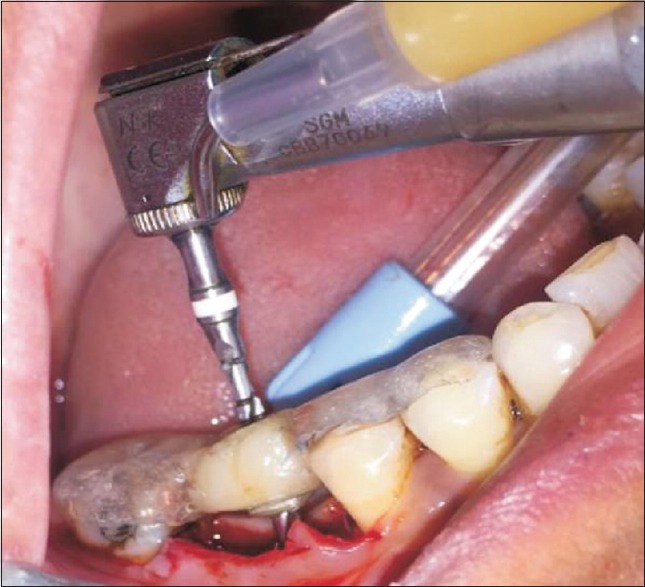
Osteotomy site preparation
Threaded root form implants (Adin Dental Implant System, Afula, Israel) were used for this study. The implant body or fixture was inserted using torque-controlled wrench [Figure 4]. The flap margins were repositioned and sutured tension free with a 3-0 braided silk suture. intraoral periapical (IOPA) radiographs were taken to assess the initial crestal bone level after implant placement. The patient was given both verbal and written instructions about postoperative routine. He/she was advised to rinse with 0.2% chlorhexidine gluconate twice daily and to take antibiotics and analgesics for three more days after surgery to minimize postoperative pain and swelling.
Figure 4.
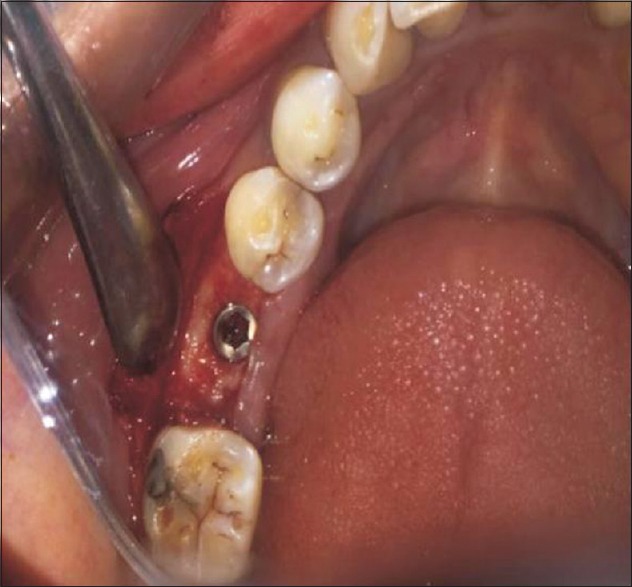
Implant in situ
Prosthodontic procedures
In Group I patient, implants were loaded within 48 h of implant placement using provisional crowns. After suturing, the surgical site was protected, using rubber dam sheet, and impression was made with addition silicone impression material (3M ESPE, USA). The transfer coping was removed from the mouth and gingival former was placed over the implant for healing. The transfer coping was attached to the implant analogue and inserted in the impression and impression was poured in die stone to transfer the implant position to the working cast for provisional restoration fabrication. Provisional restoration was cemented, by glass-ionomer cement, Type I (Gc, Gc Corporation Tokyo, Japan), within 48 h of fixture placement [Figure 5]. Suture removal was done after 1 week and the final restoration was given after 3 months of implant placement.
Figure 5.
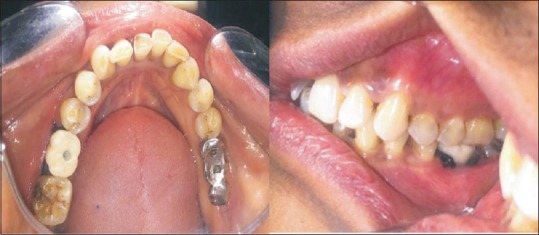
Cemented provisional restoration
In Group II patient, after allowing healing period of 3 months for the implant to get osseointegrated, phase II surgery was performed. A circular incision was placed to expose the implant. A sharp blade was used to remove all tissues coronal to the cover screw. The cover screw was removed and the head of the implant was thoroughly cleaned of any soft- or hard tissue overgrowth and healing abutments or gingival former was then placed [Figure 6]. Once the physiologic contour of soft tissue was achieved (1–2 weeks), the transfer coping was placed on the fixture and closed tray impression was made to transfer implant position and PFM restoration was fabricated. The prosthesis was cemented using glass-ionomer cement on the abutment [Figure 7]. The prosthesis in Group I patients was cemented using Type I GIC as the prosthesis was to be left undisturbed for 3 months. Use of temporary cement could have induced error due to micromovement and early decementation.
Figure 6.
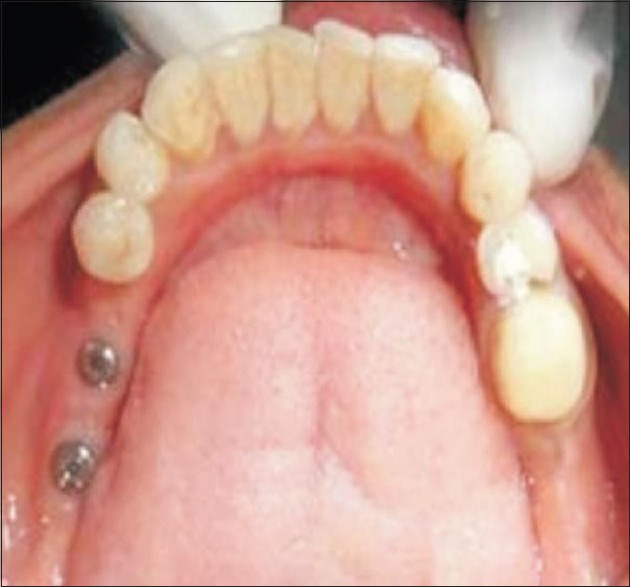
Implants with gingival former placed in Group II patient's
Figure 7.
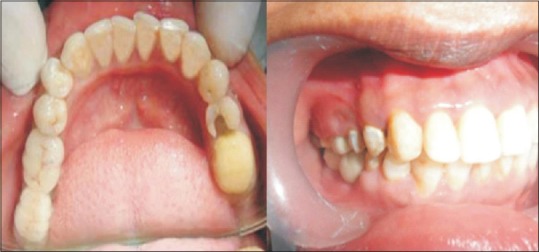
Cemented final prosthesis in Group II patient's
Radiographic evaluation and follow up
IOPA radiographs were taken for all the implant sites of selected patients. To compensate for magnification and image distortion errors, a lead grid with 1-mm2 grid pattern was affixed on to the sensor. The radiographs were standardized using the standard long cone paralleling technique with film positioning device. The follow-up was scheduled keeping the first restoration on the implants as baseline at the intervals of 1, 3, and 6 months for radiographic evaluation. The distance from the margins of the implant abutment junction to the first point of bone to implant contact was measured on mm scale [Figures 8 and 9].
Figure 8.
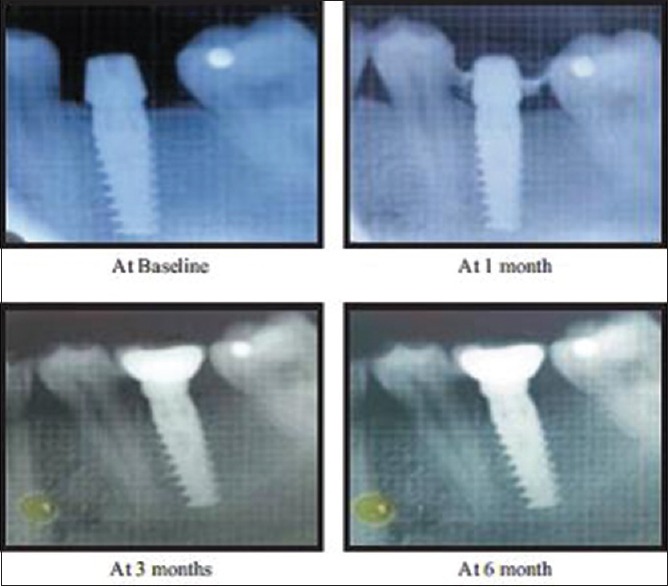
Intraoral periapicals with grid for radiographic evaluation (Immediate loading)
Figure 9.
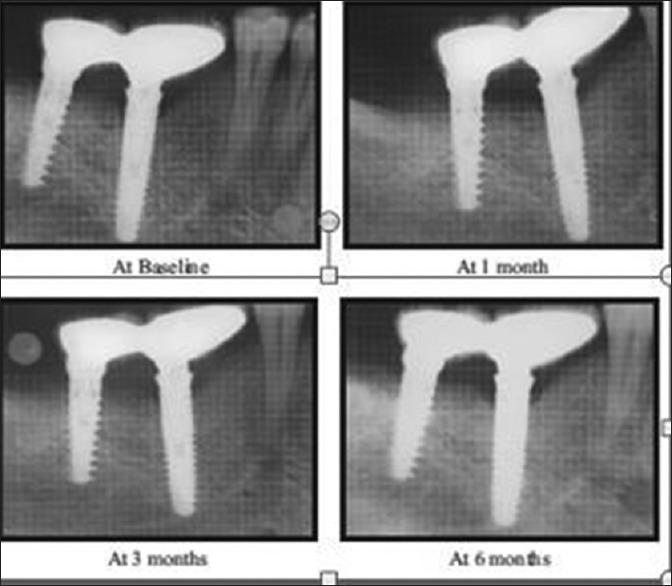
Intraoral periapicals with grid for radiographic evaluation (delayed loading)
Statistical analysis
The data collected were subjected to Friedman's test for comparison of mean radiographic bone loss at different intervals (baseline, 1, 3, and 6 months) for both Group I and Group II on mesial and distal side. For intergroup comparison, that is, for comparison of mean radiographic bone loss between Group I and Group II at different intervals on both mesial and distal side, Mann–Whitney test was used.
RESULTS
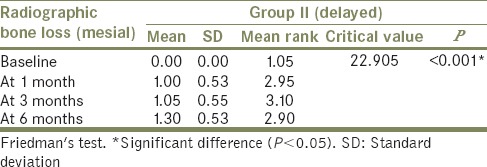
The comparison of mean radiographic bone loss (mesial side) was done among Group I (immediate loading) and Group II (delayed loading) patients between baseline, at 1 month, at 3 months, and at 6 months using the Freidman's test [Tables 2 and 3]. The result showed a significant (P < 0.05) difference in mean radiographic bone loss (mesial) when baseline (mean: 0.00) was compared to 1 month (mean: 0.90), 3 months (mean: 1.40), and 6 months (mean: 1.60) in Group I patients. Similarly, in Group II patients, significant (P < 0.05) difference in mean radiographic bone loss (mesial) when baseline (mean: 0.00) was compared to 1 month (mean: 1.00), 3 months (mean: 1.05), and 6 months (mean: 1.30) was observed. However, it was nonsignificant (P > 0.05) when 1 month was compared to 3 months and 6 months and when 3 months was compared to 6 months.
Table 2.
Comparison of mean radiographic bone loss at different intervals in Group I – mesial side
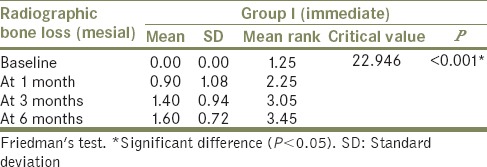
Table 3.
Comparison of mean radiographic bone loss at different intervals in Group II – mesial side
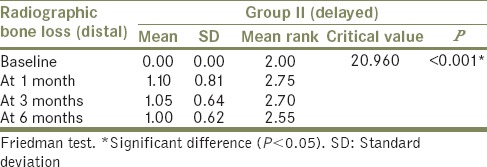
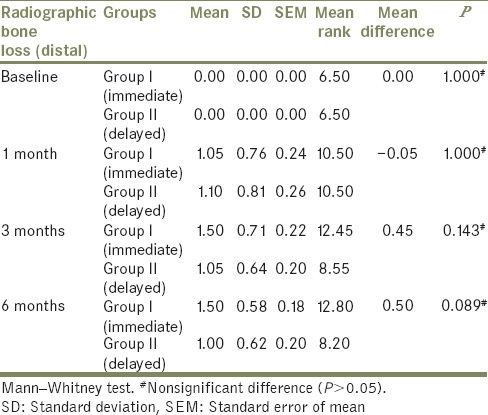
Friedman's test [Tables 4 and 5] used to compare mean radiographic bone loss (distal side) among Group I (immediate loading) and Group II (delayed loading) between baseline, at 1 month, at 3 months, and at 6 months also showed a significant (P < 0.05) difference in mean radiographic bone loss when baseline (mean: 0.00) was compared to 1 month (mean: 1.05), 3 months (mean: 1.50), and 6 months (mean: 1.50) with nonsignificant difference (P > 0.05) when 1 month was compared to 3 months and 6 months and when 3 months was compared to 6 months in Group I patient. Similar result was observed in Group II patient with mean of 0.00 at baseline, 1.10 at 1 month, 1.05 at 3 months, and 1.00 at 6 months.
Table 4.
Comparison of mean radiographic bone loss at different intervals in Group I – distal side
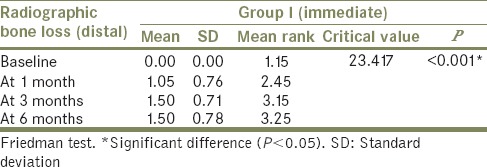
Table 5.
Comparison of mean radiographic bone loss at different intervals in Group II – distal side
The comparison of mean radiographic bone loss (mesial and distal side) was done between Group I and Group II at baseline, at 1 month, at 3 months, and at 6 months using the Mann–Whitney test [Tables 6 and 7]. The result showed no significant difference (P > 0.05) in mean radiographic bone loss between Group I and Group II at baseline, at 1 month, at 3 months, and at 6 months.
Table 6.
Comparison of mean radiographic bone loss between Group I and Group II at different months on mesial side
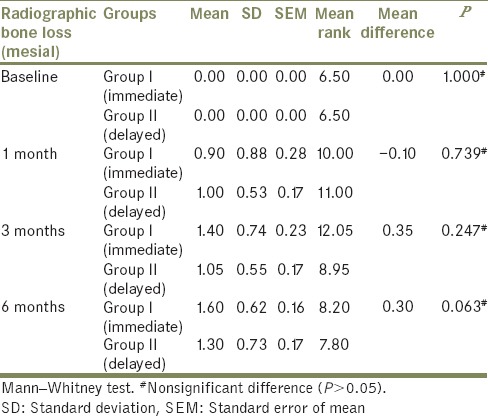
Table 7.
Comparison of mean radiographic bone loss between Group I and Group II at different months on distal side
DISCUSSION
Implant dentistry has evolved to a stage that a high implant survival rate alone, achieved by CL approach, can no longer satisfy the patients and health-care providers. Long waiting time for the implant to be osseointegrated, before the restoration can be placed, discourages patient acceptance of implant therapy. The restoration of mastication, phonetics, and esthetics that implants can provide is delayed. Different loading protocols[13,14] have thus been developed and subsequently classified as conventional (i.e., loaded at 3–6 months), early (i.e., loaded at approximately 6 weeks), or immediate (i.e., loaded at the time or within 48 h of implant placement.
The rationale for the CL protocol is to keep the implant in an undisturbed environment during the healing period.[15] It was believed that applying forces to the implant during this critical period might cause micromovement at the implant-bone surface, which in turn results in implant failure.[6] Over the past few decades, implant treatment protocols have evolved with new implant designs and surface configurations and better surgical procedures, and as a result, the period between implant placement and functional loading has been shortened.
Immediate and early loading of dental implants are techniques that are gradually gaining popularity. Such procedures are highly appreciated by the patients who can have their treatment periods drastically reduced and are able to live a normal life with minimal discomfort due to edentulism.
Various criterions have been indicated to be crucial for the success of oral implants by Albrektsson et al.[16] The most important of all is peri-implant bone levels. Trials have reported comparable marginal bone-level changes when comparing immediately versus conventionally loaded implants, but the results are contradictory.[7]
The results of this study suggested that in Group I on the mesial and distal side, significant increase in mean radiographic bone loss was seen from baseline to 1, 3, and 6 months, suggesting that there was progressive bone resorption. However, there was no significant change in mean radiographic bone loss from 1 month to 3 months and 6 months and from 3 months to 6 months, suggesting that the bone resorption stabilized after the initial period. Guruprasada et al.,[17] in 2013, have suggested that the surgical trauma and micromovement of implant caused due to the functional forces and nonfunctional forces of tongue and cheek in immediately loading the implant after its insertion may have caused the peri-implant bone loss.
This can be attributed to the fact that after loading, the occlusal stresses that implants are subjected to initiate the bone remodeling immediately after loading, that is, during the 1st month. Recent studies have shown that mechanical strain stimulates osteoblasts to produce osteoprotegerin which enhances bone deposition and downregulates osteoclastic activity as the time after loading increases.[18]
In Group II on mesial and distal side, significant increase in the mean radiographic bone loss from baseline to 1 month, 3 months, and 6 months was seen which was in accordance with a study conducted by Cardaropoli et al.,[19] in 2003, suggesting that the bulk of bone resorption, following implant surgery, occurs within the first few months, or even weeks, postimplantation. This may be due to bone remodeling, which is very active after 8 weeks of healing and presents a diverse degree of bone maturation,[20] but there was no significant change in mean radiographic bone loss from 1 month to 3 months and 6 months and from 3 months to 6 months.
In CL, initial bone loss during the postsurgery healing period caused by remodeling of bone is avoided. Furthermore, at this stage, the healing site is prevented from the action of bacteria by creating a biologic seal around the top of the implant. After the insertion of the implant and its prosthetic connection, crestal bone undergoes remodeling and resorption processes.[21]
At the time of second-stage surgery, bone is less dense and weaker than it is 6-12 months after prosthetic loading. Woven bone is unorganized and weaker than lamellar bone, which is organized and more mineralized. Lamellar bone develops several months after the woven bone repair has replaced the devitalized bone caused by surgical insertion trauma around the implant.
Furthermore, the occlusal stress levels may be high enough to cause woven bone microfracture or overload during the initial loading period, but the increase in bone strength achieved after complete mineralization and organization may be able to resist the same stress levels during the subsequent time.
As functional forces are placed on an implant, the surrounding bone can adapt to the stresses and increase its density, especially in the crestal half of implant body during the first 6 months to 1 year of loading. In a histologic and histomorphometric study of bone, Piattelli et al. reported reactions to unloaded and loaded nonsubmerged implants, the bone changed from a fine trabecular pattern after initial healing to a more dense and coarse trabecular pattern after loading, especially in the crestal half of implant interface.[22]
When Group I was compared to Group II, there was no significant change in the mean radiographic bone loss which is in accordance with the study conducted by Güncü et al.[23] in 2008 that immediate loading did not negatively affect implant stability, marginal bone levels, and peri-implant health when compared with CL. Furthermore, Schingalia et al. (2008) concluded that more peri-implant bone loss occurred in conventionally loaded implants than immediately loaded implants. They concluded that mechanical bone strain stimulation is the key factor in regulation of bone remodeling.[18] In both the groups, loading of implants was taken as the baseline and the factors that affect dynamics of the peri-implant bone such as mechanical strain and other factors that primarily initiates and regulates bone remodeling worked almost same in both the groups.
The longevity of the dental implants depends on the amount of crestal bone loss along the implant surface[24] and the crestal bone remodels after loading of implants. In the present study, in both delayed and immediate loading, there is initial bone loss which stabilizes after about a month of loading. This can be attributed to the fact that occlusal stresses, that implants are subjected to, initiates the bone remodeling immediately after loading, that is, during the 1st month. No statistically significant difference was seen in crestal bone loss on comparison of immediate loading to delayed loading. Therefore, immediate loading can be used for the benefit of the patients as it reduces the period of edentulism, is minimally invasive procedure and less complex which further decreases the discomfort and gives more psychological satisfaction to the patient.[25,26,27]
CONCLUSION
The present in vivo study assessed the influence of immediate loading and delayed loading of dental implants placed in healed sockets with respect to peri-implant bone levels. Evaluations were carried out at baseline, that is, at the time of loading, 1, 3, and 6 months for both the groups. Both immediate and delayed loading protocols showed radiographic bone loss, at both mesial and distal sides which was not found to be statistically significant. Change in radiographic bone loss in both the groups was found to be statistically significant when the baseline was compared to 1, 3, and 6 months. Within the limitations of the study, it can be concluded that there is no statistically significant difference in crestal bone loss on comparison of immediate loading to delayed loading.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Esposito M, Grusovin MG, Achille H, Coulthard P, Worthington HV. Interventions for replacing missing teeth: Different times for loading dental implants. Cochrane Database Syst Rev. 2009;1:CD003878. doi: 10.1002/14651858.CD003878.pub4. [DOI] [PubMed] [Google Scholar]
- 2.Rani I, Shetty J, Reddy V. A comparison of peri-implant strain generated by different types of implant supported prostheses. J Indian Prosthodont Soc. 2017;17:142–8. doi: 10.4103/0972-4052.203195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnitman PA, Wohrle PS, Rubenstein JE. Immediate fixed interim prostheses supported by two-stage threaded implants: Methodology and results. J Oral Implantol. 1990;16:96–105. [PubMed] [Google Scholar]
- 4.Bernard JP, Belser UC, Martinet JP, Borgis SA. Osseointegration of brånemark fixtures using a single-step operating technique. A preliminary prospective one-year study in the edentulous mandible. Clin Oral Implants Res. 1995;6:122–9. doi: 10.1034/j.1600-0501.1995.060208.x. [DOI] [PubMed] [Google Scholar]
- 5.Schnitman PA, Wöhrle PS, Rubenstein JE, DaSilva JD, Wang NH. Ten-year results for brånemark implants immediately loaded with fixed prostheses at implant placement. Int J Oral Maxillofac Implants. 1997;12:495–503. [PubMed] [Google Scholar]
- 6.Misch CE. Non-functional immediate teeth in partially edentulous patient, a pilot study of 10 consecutive cases. Compendium. 1998;19:25–36. [Google Scholar]
- 7.Engelhardt S, Papacosta P, Rathe F, Özen J, Jansen JA, Junker R, et al. Annual failure rates and marginal bone-level changes of immediate compared to conventional loading of dental implants. A systematic review of the literature and meta-analysis. Clin Oral Implants Res. 2015;26:671–87. doi: 10.1111/clr.12363. [DOI] [PubMed] [Google Scholar]
- 8.Brunski JB, Moccia AF, Jr, Pollack SR, Korostoff E, Trachtenberg DI. The influence of functional use of endosseous dental implants on the tissue-implant interface. I. Histological aspects. J Dent Res. 1979;58:1953–69. doi: 10.1177/00220345790580100201. [DOI] [PubMed] [Google Scholar]
- 9.Goodman S, Wang JS, Doshi A, Aspenberg P. Difference in bone ingrowth after one versus two daily episodes of micromotion: Experiments with titanium chambers in rabbits. J Biomed Mater Res. 1993;27:1419–24. doi: 10.1002/jbm.820271109. [DOI] [PubMed] [Google Scholar]
- 10.Goodman SB. The effects of micromotion and particulate materials on tissue differentiation. Bone chamber studies in rabbits. Acta Orthop Scand Suppl. 1994;258:1–43. doi: 10.3109/17453679409155227. [DOI] [PubMed] [Google Scholar]
- 11.Vidyasagar L, Aspe P. Biological response to dental implant loading/Overloading? Implant overloading: Empiricism or science. Stomatologija Baltic Dent Maxillofac J. 2003;5:83–9. [Google Scholar]
- 12.Szmukler-Moncler S, Salama H, Reingewirtz Y, Dubruille JH. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J Biomed Mater Res. 1998;43:192–203. doi: 10.1002/(sici)1097-4636(199822)43:2<192::aid-jbm14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.Gallucci GO, Benic GI, Eckert SE, Papaspyridakos P, Schimmel M, Schrott A, et al. Consensus statements and clinical recommendations for implant loading protocols. Int J Oral Maxillofac Implants. 2014;29(Suppl):287–90. doi: 10.11607/jomi.2013.g4. [DOI] [PubMed] [Google Scholar]
- 14.Wang HL, Ormianer Z, Palti A, Perel ML, Trisi P, Sammartino G, et al. Consensus conference on immediate loading: The single tooth and partial edentulous areas. Implant Dent. 2006;15:324–33. doi: 10.1097/01.id.0000246248.55038.3a. [DOI] [PubMed] [Google Scholar]
- 15.Brånemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–32. [PubMed] [Google Scholar]
- 16.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 17.Guruprasad M, Thapliyal GK, Pawar GR. A comparative analysis of periimplant bone levels of immediate and conventionally loaded implants. Med J Armed Forces India. 2013;69:41–7. doi: 10.1016/j.mjafi.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schincaglia GP, Marzola R, Giovanni GF, Chiara CS, Scotti R. Replacement of mandibular molars with single-unit restorations supported by wide-body implants: Immediate versus delayed loading. A randomized controlled study. Int J Oral Maxillofac Implants. 2008;23:474–80. [PubMed] [Google Scholar]
- 19.Cardaropoli G, Wennström JL, Lekholm U. Peri-implant bone alterations in relation to inter-unit distances. A 3-year retrospective study. Clin Oral Implants Res. 2003;14:430–6. doi: 10.1034/j.1600-0501.2003.00895.x. [DOI] [PubMed] [Google Scholar]
- 20.Lopes Cde C, König B., Júnior Histological findings of bone remodeling around smooth dental titanium implants inserted in rabbit's tibias. Ann Anat. 2002;184:359–62. doi: 10.1016/s0940-9602(02)80056-5. [DOI] [PubMed] [Google Scholar]
- 21.Hermann JS, Buser D, Schenk RK, Higginbottom FL, Cochran DL. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000;11:1–1. doi: 10.1034/j.1600-0501.2000.011001001.x. [DOI] [PubMed] [Google Scholar]
- 22.Piattelli A, Manzon L, Scarano A, Paolantonio M, Piattelli M. Histologic and histomorphometric analysis of the bone response to machined and sandblasted titanium implants: An experimental study in rabbits. Int J Oral Maxillofac Implants. 1998;13:805–10. [PubMed] [Google Scholar]
- 23.Güncü MB, Aslan Y, Tümer C, Güncü GN, Uysal S. In-patient comparison of immediate and conventional loaded implants in mandibular molar sites within 12 months. Clin Oral Implants Res. 2008;19:335–41. doi: 10.1111/j.1600-0501.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh P, Garge HG, Parmar VS, Viswambaran M, Goswami MM. Evaluation of implant stability and crestal bone loss around the implant prior to prosthetic loading: A six month study. J Ind Prosthodont Soc. 2006;6:33–7. [Google Scholar]
- 25.Lazzara RJ. Immediate implant placement into extraction sites: Surgical and restorative advantages. Int J Periodontics Restorative Dent. 1989;9:332–43. [PubMed] [Google Scholar]
- 26.Sczwartz-Ariad D, Chaushu G. Placement of implants into fresh extraction sites: 4-7 years retrospective evaluation of 95 immediate implants. J Periodontol. 1997;68:1110–6. doi: 10.1902/jop.1997.68.11.1110. [DOI] [PubMed] [Google Scholar]
- 27.Hahn J. Single-stage, immediate loading, and flapless surgery. J Oral Implantol. 2000;26:193–8. doi: 10.1563/1548-1336(2000)026<0193:SILAFS>2.3.CO;2. [DOI] [PubMed] [Google Scholar]


