Abstract
A high SAMe-TT2R2 score predicted poor warfarin control and adverse events among atrial fibrillation patients. However, the SAMe-TT2R2 score has not been well validated in venous thromboembolism (VTE) patients. A cohort of 1943 warfarin-treated patients with acute VTE was analyzed to correlate the SAMe-TT2R2 score with time in therapeutic range (TTR) and clinical adverse events. A TTR <60% was more frequent among patients with a high (>2) versus low (0–1) SAMe-TT2R2 score (63.4% vs 52.3%, p<0.0001). A high SAMe-TT2R2 score (>2) correlated with increased overall adverse events (7.9 vs 4.5 overall adverse events/100 patient years, p=0.002), driven primarily by increased recurrent VTE rates (4.2 vs 1.5 recurrent VTE/100 patient years, p=0.0003). The SAMe-TT2R2 score had a modest predictive ability for international normalized ratio (INR) quality and adverse clinical events among warfarin-treated VTE patients. The utility of the SAMe-TT2R2 score to guide clinical decision-making remains to be investigated.
Keywords: anticoagulants, pulmonary embolism, venous thromboembolism (VTE)
Introduction
The emergence of direct oral anticoagulants (DOACs) as an alternative to vitamin K antagonists (VKA) has altered the therapeutic options for patients with non-valvular atrial fibrillation (NVAF) and venous thromboembolism (VTE).1,2 In fact, society guidelines now recommend DOACs over VKA as the initial therapy for VTE and as an alternative agent for NVAF patients with a poor quality of anticoagulation.3,4 Clinicians and patients are therefore faced with the decision of selecting an anticoagulation plan while balancing the risks of bleeding, thrombosis, reversibility and patient preference.5,6
A proposed method to guide the selection of an anticoagulant is to predict the quality of VKA therapy using the SAMe-TT2R2 score (Table 1).7–9 This prediction score was derived from clinical trial data comparing rate and rhythm strategy in NVAF patients.10 It has since been validated in prospective cohorts and has demonstrated the ability to risk stratify NVAF patients likely to have poor international normalized ratio (INR) control.11–14 Compared to a low SAMe-TT2R2 score (0–1), a high score (>2) predicted lower quality anticoagulation (lower TTR) and increased risk of adverse outcomes.15 TTR, computed by the Rosendaal linear interpolation method, quantifies the percentage of time spent by a patient within a therapeutic INR range and is used to assess quality of anticoagulation.16 Identifying patients who will have poor INR control by SAMe-TT2R2 score can assist clinicians to monitor high-risk patients closely or alternatively to select a DOAC as initial therapy.17 In fact, a few society guidelines and expert opinion documents endorsed the use of the SAMe-TT2R2 score as a method for anticoagulation selection in patients with newly diagnosed NVAF.18,19
Table 1.
SAMe-TT2R2 variables.
| Variables | Points | |
|---|---|---|
| S | Sex – female | 1 |
| A | Age < 60 years | 1 |
| Me | Medical history > 2 comorbiditiesa | 1 |
| T | Treatment medications (e.g. amiodarone) | 1 |
| T2 | Tobacco use | 2 |
| R2 | Race – non-Caucasian | 2 |
| Maximum points | 8 |
Me, one point is allotted if more than two of the following conditions are present: diabetes, hypertension, renal disease or hepatic disease, pulmonary disease, congestive heart failure, coronary artery disease, peripheral vascular disease or previous stroke.
The predictive ability of the SAMe-TT2R2 score for a low quality of anticoagulation in the VTE population, however, is not well described. In this study, we validated the SAMe-TT2R2 score in real-world patients undergoing anticoagulation for acute VTE. Furthermore, we investigated adverse clinical outcomes and additional clinical characteristics to improve the performance of the SAMe-TT2R2 score for predicting a poor quality of anticoagulation.
Methods
MAQI2 collaborative
The Michigan Anticoagulation Quality Improvement Initiative (MAQI2) is a Blue Cross and Blue Shield of Michigan/Blue Care Network (BCBSM/BCN)-funded multicenter network of anticoagulation clinics in the state of Michigan. Full details about MAQI2 have been described previously.20 MAQI2 collects de-identified patient data on various clinical parameters with a goal of identifying practice patterns, improving patient safety and outcomes, and collaborating with quality improvement initiatives. MAQI2 was formed in 2008 and data collection began in 2009. Currently, there are six hospitals in Michigan participating in the program. All data abstractors undergo training, and each center undergoes regular audits to ensure high-quality data collection and agreement with pre-defined data element definitions.
Patient selection
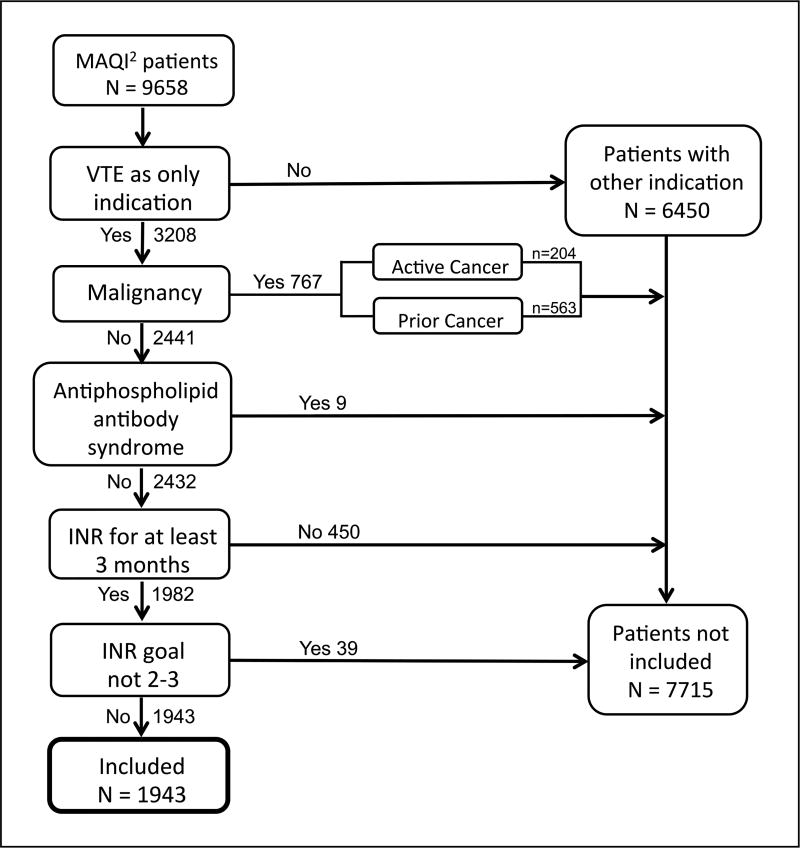
Patients with acute VTE treated with warfarin as the primary indication at a participating MAQI2 center were included in the trial. Individuals with malignancy were excluded from the trial because low molecular weight heparin is the standard of care from current treatment guidelines.3 Additional exclusion criteria include patients treated with warfarin and had regular INR lab draws for less than the 3-month standard for VTE treatment, anti-phospholipid antibody syndrome, and an INR goal outside of 2–3 (Figure 1).
Figure 1.
Flow chart showing patient selection criteria. Patients without INR for 3 months included new patients (n=39), patients no longer requiring anticoagulation (n=234), change to non-warfarin anticoagulation (n=91) and lost to follow up (n=86). (MAQI2, Michigan Anticoagulation Quality Improvement Initiative; VTE, venous thromboembolism; INR, international normalized ratio.)
Calculation of the SAMe-TT2R2 score
As described in the original article, one point was assigned for female sex, age less than 60 years, presence of two or more medical comorbid conditions, and therapy with medications known to interact with warfarin (i.e. amiodarone).7 Two points were assigned each for concurrent tobacco use and non-Caucasian ethnicity. Medical history was allotted a point if more than two of the following conditions were present: diabetes, hypertension, renal disease or hepatic disease, pulmonary disease, congestive heart failure, coronary artery disease, peripheral vascular disease or previous stroke (Table 1). All elements were calculated at the time of patient enrollment in the anticoagulation clinic.
Data collection
Demographic information, medications and medical comorbid diseases were abstracted from the patient’s chart at the time of warfarin initiation. Laboratory values, including INR, were obtained from patient charts to determine the quality of anticoagulation. The Rosendaal linear interpolation method was used to determine TTR.16
Outcomes
The SAMe-TT2R2 score was analyzed both as a categorical and as a continuous variable for TTR. Clinical outcomes were compared dichotomously between 0 and 2 and >2 based on recent literature regarding the decision-making algorithm using the SAMe-TT2R2 score.19,21 A logistic regression model was used to determine the discriminative ability of the SAMe-TT2R2 score for TTR below 60%. A cut-off of <60% was used for this analysis based on prior studies establishing TTR below 60% as a marker of poor anticoagulation.22
Clinical events were pre-defined using a standardized data abstraction form and data dictionary. All events were abstracted from the medical chart as denoted by the clinical teams (e.g. anticoagulation clinic staff, primary care or specialty physicians, and emergency room providers). These events were manually abstracted by trained data abstractors (not reliant on billing codes) and adverse events were randomly audited by the coordinating center to assure accuracy. Prior VTE, heavy alcohol use and weight were added in a second logistic regression analysis to assess for improved performance characteristic of the SAMe-TT2R2 score.23 Adverse clinical events included: (1) recurrent VTE, a new deep vein thrombosis (DVT) or pulmonary embolism (PE); (2) International Society on Thrombosis and Haemostasis (ISTH) major bleeding; and (3) overall adverse events, a summation of recurrent VTE and ISTH major bleeding. Our use of ISTH major bleeding was consistent with the 2005 consensus definition.24 Patients were followed only through the duration of warfarin therapy. Therefore, follow-up time is equivalent to length of warfarin treatment.
Statistical analysis
A chi-squared test was used to test the association between SAMe-TT2R2 and clinical variables including age, sex, non-white ethnicity, mean TTR, TTR <60%, tobacco use, treatment medications, medical history, weight, prior VTE, and alcohol use. A logistic regression model was developed to determine the predictive ability of the SAMe-TT2R2 for a poor quality of anticoagulation (<60%). A second logistic regression model with the addition of age, alcohol use and weight was created to assess if the variables improved the discriminative ability of the SAMe-TT2R2 score. To analyze the difference in clinical adverse events rate between SAMe-TT2R2 groups, a generalized linear model was developed.
Sensitivity analysis
To better explore the clinical utility of the SAMe-TT2R2 score, we assessed its predictive ability across a range of TTR thresholds (<60%, <65%, and <70%). C-statistics were compared qualitatively between these three thresholds. Given that warfarin is a standard of care option in patients with a history of cancer that is not active or currently being treated, we performed a second sensitivity analysis to explore the impact of patients with a prior history of malignancy (n=563) who were treated with warfarin for VTE therapy on the predictive ability of the SAMe-TT2R2 score for a TTR <60%.
Results
Baseline characteristics
Our study included 1943 patients (average age 57.1 years, 52.3% female) treated with VKA for acute VTE (Table 2). Patients were well distributed among low (34.2%), borderline (22.2%) and high (43.5%) SAMe-TT2R2 scores. Compared to the cohort with a low SAMe-TT2R2 score, younger patients, female sex, tobacco users and non-Caucasian individuals were more frequently distributed in the borderline and higher SAMe-T2TR2 cohorts.
Table 2.
Patient demographic and low TTR divided by SAMe-TT2R2 level.
| Indication: VTE only (n=1225) | |||||
|---|---|---|---|---|---|
|
|
|||||
| SAMe-TT2R2 Low (0–1) n = 665 (34.2%) |
SAMe-TT2R2 Borderline (2) n = 432 (22.2%) |
SAMe-TT2R2 Higher (>2) n = 846 (43.5%) |
Total n = 1943 |
p-value | |
| Age, years, mean ± SD | 61.8 ± 15.7 | 54.1 ± 16.9 | 55.0 ± 17.0 | 57.1 ± 16.9 | |
| Age <60 years | 255 (38.4) | 277 (64.1) | 528 (62.4) | 1060 (54.6) | <0.0001 |
| Female | 217 (32.6) | 299 (69.2) | 501 (59.2) | 1017 (52.3) | <0.0001 |
| Non-white | 0 | 29 (6.7) | 472 (55.8) | 501 (25.8) | <0.0001 |
| Tobacco use | 0 | 80 (18.5) | 495 (58.5) | 575 (29.6) | <0.0001 |
| Treatment, amiodarone | 0 | 8 (1.9) | 14 (1.6) | 22 (1.1) | 0.003 |
| Medical history | 38 (5.7) | 62 (14.4) | 156 (18.4) | 256 (13.2) | <0.0001 |
| Mean weight, lbs ± SD | 204.5 ± 54.4 | 193.7 ± 52.1 | 201.0 ± 63.7 | 200.6 ± 58.2 | |
| Prior VTE | 185 (27.8) | 98 (22.7) | 205 (24.3) | 488 (25.1) | 0.12 |
| Alcohol use | 21 (3.2) | 20 (4.6) | 65 (7.7) | 106 (5.5) | 0.004 |
| Length of follow up, years, median ± IQR | 0.56 ± 1.13 | 0.56 ± 0.92 | 0.55 ± 0.91 | 0.56 ± 0.96 | |
| TTR <60% | 348 (52.3) | 233 (53.9) | 536 (63.4) | 1117 (57.6) | <0.0001 |
Data presented as number (%), unless otherwise noted in the row.
TTR, time in therapeutic range (linear interpolation); VTE, venous thromboembolism; IQR, interquartile range.
Predictive ability of SAMe-TT2R2 for poor quality of anticoagulation
As the SAMe-TT2R2 increased, the mean TTR declined (Table 3). Compared to a low (0–1) SAMe-TT2R2 score, a high score (>2) was associated with both lower TTR (50% vs 57%) and an increased proportion of patients with TTR <60% (63.4% vs 52.3%, p<0.0001).
Table 3.
Relationship between continuous and categorical SAMe-TT2R2 scores with TTR.
| n | TTR (± SD) | |
|---|---|---|
| Continuous SAMe-TT2R2 | ||
| 0 | 155 | 60 ± 20 |
| 1 | 510 | 56 ± 21 |
| 2 | 432 | 55 ± 22 |
| 3 | 431 | 51 ± 23 |
| 4 | 289 | 49 ± 22 |
| >5 | 126 | 48 ± 22 |
| Categorical scale | ||
| Low (0–1) | 665 | 57 ± 21 |
| Borderline (2) | 432 | 55 ± 22 |
| High (>2) | 846 | 50 ± 23 |
TTR, time in therapeutic range (linear interpolation).
The SAMe-TT2R2 score was associated with increased odds of a poor quality of warfarin (TTR <60%) care (odds ratio (OR) 1.18, 95% confidence interval (CI) 1.11–1.26, p<0.0001). In a sensitivity analysis, the discriminative ability of the SAMe-TT2R2 did not vary significantly for predicting TTR <60%, <65%, or <70% with c-statistics of 0.61, 0.65, and 0.65, respectively.
Individual components of the SAMe-TT2R2 score were analyzed for their predictive ability for TTR <60% and no individual factor was independently associated with predicting poor anticoagulation (Table 4). Weight, prior VTE, and alcohol use, risk factors not included in the SAMe-TT2R2 score, were statistically significant predictors of low TTR (<60%) in the combined model (Table 4). With the addition of these variables, the performance characteristic minimally improved, with a c-statistic = 0.64, compared to the original SAMe-TT2R2 score. Finally, a third logistic regression model incorporating only age <60, medical history, tobacco use, weight, prior VTE, and alcohol use was analyzed and yielded a c-statistic = 0.63, but without meaningful change in the OR point estimates (online Supplementary Appendix). In a sensitivity analysis to explore the impact of including patients with a history of malignancy (non-active) on the predictive ability of the SAMe-TT2R2 for a TTR <60%, there was no meaningful change in the OR point estimate (1.18, 95% CI 1.11–1.25) or the discriminatory ability (c-statistic = 0.59).
Table 4.
Logistic regression for predictability of SAMe-TT2R2 on low TTR (with control of site).
| OR (95% CI) | p-value | AUC | |
|---|---|---|---|
| Model 1: | |||
| SAMe-TT2R2 score | 1.18 (1.11–1.26) | <0.0001 | 0.61 |
| Model 2: | |||
| Age <60 years | 0.55 (0.45–0.68) | <0.0001 | 0.64 |
| Amiodarone | 0.56 (0.22–1.41) | 0.22 | |
| Medical history | 0.67 (0.51–0.92) | 0.01 | |
| Female | 0.95 (0.78–1.16) | 0.58 | |
| Non-Caucasian | 0.91 (0.81–1.02) | 0.11 | |
| Tobacco use | 0.93 (0.81–1.01) | 0.07 | |
| Weight (per 10 lbs) | 1.003 (1.001–1.004) | 0.003 | |
| Prior VTE | 1.21 (0.97–1.51) | 0.09 | |
| Alcohol use | 0.48 (0.30–0.78) | 0.003 | |
| Insurance status | 1.71 (0.64–4.58) | 0.28 |
TTR, time in therapeutic range; OR, odds ratio; CI, confidence interval; AUC, area under the receiver operator curve; VTE, venous thromboembolism.
Clinical outcomes
In a dichotomous comparison of the SAMe-TT2R2 score, the rate of overall adverse events was statistically higher in patients with a score >2 compared to 0–2 (7.9 vs 4.5 overall adverse events/100 patient-years, p=0.002; Table 5). This was driven by an increased rate of recurrent VTE in patients with a score >2 (4.2 vs 1.5 recurrent VTE/100 patient-years, p=0.0003). While the point estimate for ISTH major bleeding (3.7 vs 3.0 major bleeding/100 patient-years, p=0.43) was numerically increased in patients with a SAMe-TT2R2 score >2 compared to 0–2, this was not statistically significant. Rates of overall adverse events and recurrent VTE increased as SAMe-TT2R2 scores increased (online Supplementary Appendix).
Table 5.
Adverse clinical outcomes with low (0–2) and high (>2) SAMe-TT2R2 score.
| SAMe-TT2R2 | p-value | ||
|---|---|---|---|
|
|
|||
| 0–2 | >2 | ||
| No. of adverse clinical outcomes | 57 | 69 | – |
| Recurrent VTE rates (per 100 patient-years) | 1.5 | 4.2 | 0.0003 |
| ISTH major bleeding rates (per 100 patient-years) | 3.0 | 3.7 | 0.43 |
| Overall adverse events rates (per 100 patient-years) | 4.5 | 7.9 | 0.002 |
VTE, venous thromboembolism; ISTH, International Society on Thrombosis and Haemostasis.
Discussion
In a cohort of ‘real-world’ patients with acute VTE treated with VKA, we analyzed the relationship between SAMe-TT2R2 and quality of anticoagulation, as measured by TTR and adverse clinical outcomes. Our principal finding is that a high SAMe-TT2R2 score was associated with a lower TTR and an increased proportion of patients with TTR <60% compared to patients with a low SAMe-TT2R2 score. In addition, a score >2 correlated with an increased rate of recurrent VTE and overall adverse events. While an increased rate of ISTH major bleeding was observed, this did not reach statistical significance. Finally, weight, alcohol use, and prior VTE were identified as additional clinical parameters associated with a poor quality of anticoagulation. A separate model incorporating these clinical factors led to minimal improvements in the discriminatory ability for poor quality VKA care.
The safety and efficacy of VKA for the treatment of VTE depends vastly on the quality of anticoagulation. Observational studies demonstrate increased rates of adverse clinical outcomes, including recurrent thrombosis, bleeding and death, with low TTR.25,26 The emergence of DOACs has altered the therapeutic options for VTE. Numerous clinical trials have now demonstrated that DOACs are non-inferior to VKA for VTE patients with the benefit of a favorable risk profile.27–32 Anticoagulant selection between a DOAC and VKA often requires judicious consideration of medical comorbidities, bleeding risk and patient preference. The SAMe-TT2R2 utilizes simple clinical parameters and has been suggested as a tool to assist in anticoagulation selection. Our study offers external validation of SAMe-TT2R2 for the prediction of VKA quality for a population of real-world patients with acute VTE. Of particular importance is the inclusion of patients with acute VTE because poor anticoagulation during this phase has been associated with worse long-term outcomes and recurrent VTE.33
Our study corroborates with prior studies demonstrating that a high SAMe-TT2R2 (>2) score identifies patients at risk of having poor anticoagulation. In a large prospective cohort of NVAF patients, Poli et al. demonstrated a decrement in mean TTR from 74% to 68% as a patient’s SAMe-TT2R2 score increased.13 Aside from one small trial with a high quality of anticoagulation,34 studies have largely confirmed this finding for patients with NVAF.11–15 Palareti et al. first validated the SAMe-TT2R2 in acute VTE patients, demonstrating worse anticoagulation care in patients with a high SAMe-TT2R2 score and a c-statistic = 0.52.35 In our study, SAMe-TT2R2 has a modest predictive ability for a poor quality of anticoagulation (TTR <60%) (c-statistic = 0.61), and the performance characteristic did not change significantly at higher cut-offs of TTR. Prior studies in NVAF and VTE have similar performance characteristics of the SAMe-TT2R2 score and consistently demonstrate an association of higher score with a poor quality of anticoagulation, with a c-statistic range of 0.52–0.57.11,35,36
Rather than developing a unique model to predict anticoagulation quality in warfarin-treated VTE patients, we validated the existing SAMe-TT2R2 score for two reasons. First, many variables including female sex, younger age, medical comorbidities, tobacco use, and non-Caucasian ethnicity have been associated with a poor quality of anticoagulation in both VTE and NVAF patient populations.37,38 Second, a single universal score would assist practitioners caring for patients with varying indications for warfarin therapy. VTE-specific predictors of poor warfarin quality, including weight, alcohol use, and prior VTE, were added to the existing SAMe-TT2R2 elements.23 However, a separate model that incorporated these factors only marginally improved the ability to predict TTR <60% (c-statistic = 0.64 vs 0.61). Further investigation is warranted to determine if VTE-specific factors can improve the existing SAMe-TT2R2 model for warfarin-treated acute VTE patients.
An important finding in our analysis is the association between high SAMe-TT2R2 with clinically relevant outcomes. Rates of recurrent VTE and overall adverse events were significantly higher in patients with a SAMe-TT2R2 score >2 compared to 0–2. While the point estimates for ISTH major bleeding were increased in patients with a score >2, this did not reach statistical significance. To our knowledge, this is the first validation of the SAMe-TT2R2 in VTE patients demonstrating significant adverse clinical outcomes. The correlation between the SAMe-TT2R2 score and adverse clinical events in NVAF, and now VTE, strengthens the clinical utility of this model to assist clinicians in mitigating undue harms associated with anticoagulation. These findings suggest that acute VTE patients can be incorporated into the decision-making algorithm previously suggested for anticoagulation-naïve NVAF patients.14,19,21
Strengths and limitations
Our study has numerous strengths including a large study population, broad range of SAMe-TT2R2 scores and evaluation of clinically meaningful outcomes. We demonstrated for the first time that SAMe-TT2R2 is associated with a significantly increased risk of overall adverse events in acute VTE patients. However, important limitations must be considered. The main limitation of the study is a retrospective design, which limits the ability to account for unmeasured confounders and precludes the study of the SAMe-TT2R2 score in clinical decision-making. Second, we excluded patients with malignancy. However, in a sensitivity analysis that included patients with a prior history of malignancy (not active malignancy), there was no meaningful change in the predictive ability or discriminatory function of the SAMe-TT2R2 score for warfarin-treated VTE patients in our cohort. A similar frequency of warfarin use for the treatment of malignancy-associated VTE has been reported from a separate patient registry.39 While malignancy has been independently associated with poor anticoagulation quality, treatment with low molecular weight heparin is the standard of care proposed by major society guidelines.3 Additional risk factors such as temporary warfarin interruption for invasive procedures, distance from anticoagulation center, insurance status and educational level could not be assessed from the MAQI2 database but can be hypothesized to impact anticoagulation quality. While the overall warfarin quality from our regional anticoagulation centers was lower than studies that have validated the SAMe-TT2R2 score, this may reflect greater distribution of patients with a high SAMe-TT2R2 score in our cohort, who would be predicted to have lower TTR. Furthermore, mean TTR for most atrial fibrillation patients in the United States is <55–60% (even lower in the first 6 months), and would likely be the same for VTE patients.40 Finally, clinical evidence supports improved TTR with increased duration of anticoagulation and dose adjustments. However, we did not adjust for these potential confounders because the primary utility of the SAMe-TT2R2 score is for risk stratification of warfarin quality at the time of warfarin initiation.
Conclusion
In conclusion, the SAMe-TT2R2 serves as a simple clinical tool that can risk stratify patients who are more likely to have poor anticoagulation care on VKA. An ability to identify such patients can guide a clinician towards selecting DOAC initially or monitoring high-risk patients closely and modifying factors contributing to poor INR control. A high SAMe-TT2R2 score is associated with a poor quality of anticoagulation and increased rates of overall adverse events driven by elevated rates of recurrent VTE. Prospective studies utilizing the SAMe-TT2R2 score for clinical decision-making between VKA and DOACs are required before widespread adoption of this model, especially in VTE patients.
Supplementary Material
Acknowledgments
EKR – consultant: Janssen, ACP; board member: Anticoagulation Forum. SA − consulting fees/honoraria: Kona, Trice Orthopedics, Micardia; ownership/partnership/principal: Biostar Ventures, Ablative Solutions; research/research grants: Boston Scientific Watchman, Abbott Absorb trial. SK – consultant: Boehringer-Ingelheim, Janssen, Daiichi Sankyo, Bristol-Myers Squibb/Pfizer, Portola; speaker’s bureau: Janssen, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, CSL Behring. JBF – consultant: Merck, Bristol-Myers Squibb/Pfizer, Sanofi-Aventis, Janssen Pharmaceuticals; research grants: Fibromuscular Disease Society of America, Blue Cross Blue Shield of Michigan. GDB – consultant: Portola, Aralez; research grants: Blue Cross Blue Shield of Michigan, Bristol- Myers Squibb/Pfizer.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: GDB is supported on the NHLBI grant T32-HL007853. The Michigan Anticoagulation Quality Improvement Initiative (MAQI2) is supported by the Blue Cross Blue Shield of Michigan/Blue Care Network.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AK, XK, BH, JK, GDK, MWM – none.
The supplementary material is available at http://vmj.sagepub.com/supplemental
References
- 1.Kirley K, Qato DM, Kornfield R, et al. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes GB, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–1305. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearon C, Akl EA, Ormelas J, et al. Antithrombotic Therapy for VTE Disease CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Hulle T, Kooiman J, Den Exter PL, et al. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: A systemic review and meta-analysis. J Thromb Haemost. 2014;12:320–328. doi: 10.1111/jth.12485. [DOI] [PubMed] [Google Scholar]
- 6.Zolfaghari S, Harenberg J, Froelich L, et al. Development of a tool to identify patients’ preference for vitamin K antagonist or direct oral anticoagulant therapy. Semin Thromb Hemost. 2014;40:121–128. doi: 10.1055/s-0033-1361940. [DOI] [PubMed] [Google Scholar]
- 7.Apostolakis S, Sullivan R, Olshansky B, et al. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: The SAMe-TT2R2 score. Chest. 2013;144:1555–1563. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
- 8.Fauchier L, Poli D, Olshansky B. The SAMe-TT2R2 score and quality of anticoagulation in AF. Can we predict which patient benefits from anticoagulation? Thromb Haemost. 2014;114:657–659. doi: 10.1160/TH15-06-0518. [DOI] [PubMed] [Google Scholar]
- 9.Esteve-Pastor MA, Roldán V, Valdés M, et al. The SAMe-TT2R2 score and decision-making between a vitamin K antagonist or a non-vitamin K antagonist oral anticoagulant in patients with atrial fibrillation. Expert Rev Cardiovas Ther. 2016;14:177–187. doi: 10.1586/14779072.2016.1116941. [DOI] [PubMed] [Google Scholar]
- 10.AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Ortiz M, Bertomeu V, Cequier A, et al. Validation of the SAMe-TT2R2 score in a nationwide population of non-valvular atrial fibrillation patients on vitamin K antagonists. Thromb Haemost. 2015;114:695–701. doi: 10.1160/TH15-02-0169. [DOI] [PubMed] [Google Scholar]
- 12.Gallego P, Roldan V, Marin F, et al. SAMe-TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am J Med. 2014;127:1083–1088. doi: 10.1016/j.amjmed.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Poli D, Antonucci E, Testa S, et al. A prospective validation of the SAMe-TT2R2 score: How to identify atrial fibrillation patients who will have good anticoagulation control on warfarin. Intern Emerg Med. 2014;9:443–447. doi: 10.1007/s11739-014-1065-8. [DOI] [PubMed] [Google Scholar]
- 14.Roldan V, Cancio S, Galvez J, et al. The SAMe-TT2R2 score predicts poor anticoagulation control in AF patients: A prospective ‘real-world’ inception cohort study. Am J Med. 2015;128:1237–1243. doi: 10.1016/j.amjmed.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Haguenoer K, Saint-Etienne C, et al. Relationship of the SAMe-TT2R2 score to poor-quality anticoagulation, stroke, clinically relevant bleeding, and mortality in patients with atrial fibrillation. Chest. 2014;146:719–726. doi: 10.1378/chest.13-2976. [DOI] [PubMed] [Google Scholar]
- 16.Rosendaal F, Cannegieter S, Van Der Meer F, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 17.Fauchier L, Angoulvant D, Lip GY. The SAMe-TT2R2 score and quality of anticoagulation in atrial fibrillation: A simple aid to decision making on who is suitable (or not) for vitamin K antagonists. Eurospace. 2015;17:671–673. doi: 10.1093/europace/euv088. [DOI] [PubMed] [Google Scholar]
- 18.National Clinical Guideline Centre (UK) Atrial fibrillation: The management of atrial fibrillation. London: National Institute for Health and Care Excellence (UK); 2014. [PubMed] [Google Scholar]
- 19.Lip GY, Lane DA. Stroke prevention in atrial fibrillation a systematic review. JAMA. 2015;313:1950–1962. doi: 10.1001/jama.2015.4369. [DOI] [PubMed] [Google Scholar]
- 20.Barnes GD, Kaatz S, Gu X, et al. Warfarin use in atrial fibrillation patients at low risk for stroke: Analysis of the Michigan Anticoagulation Quality Improvement Initiative (MAQI2) J Thromb Thrombolysis. 2014;37:171–176. doi: 10.1007/s11239-013-0934-8. [DOI] [PubMed] [Google Scholar]
- 21.Proietti M, Lip GY. Simple decision-making between a vitamin K antagonist and a non-vitamin K antagonist oral anticoagulation: Using the SAMe-TT2R2 score. Eur Heart J Cardiovasc Pharmacother. 2015;1:150–152. doi: 10.1093/ehjcvp/pvv012. [DOI] [PubMed] [Google Scholar]
- 22.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 23.Kooistra HA, Gebel M, Sahin K, et al. Independent predictors of poor vitamin K antagonist control in venous thromboembolism patients. Data from the EINSTEIN-DVT and PE studies. Thromb Haemost. 2015;114:1136–1143. doi: 10.1160/TH14-12-1033. [DOI] [PubMed] [Google Scholar]
- 24.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher AM, de Vries F, Plumb JM, et al. Quality of INR control and outcomes following venous thromboembolism. Clin Appl Thromb Hemost. 2012;18:370–378. doi: 10.1177/1076029611426139. [DOI] [PubMed] [Google Scholar]
- 26.Veeger MJ, Piersma-Wichers M, Tijsen JG, et al. Individual time within target range in patients treated with vitamin K antagonists: Main determinant of quality of anticoagulation and predictor of clinical outcome. A retrospective study of 2300 consecutive patients with venous thromboembolism. Br J Haematol. 2005;128:513–519. doi: 10.1111/j.1365-2141.2004.05348.x. [DOI] [PubMed] [Google Scholar]
- 27.Schulman S, Kaeron C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 28.Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 29.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 30.Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 31.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 32.The Hokusai VTE Investigators. Endoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 33.Palareti G, Legnani C, Cosmi B, et al. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3:955–961. doi: 10.1111/j.1538-7836.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 34.Skov J, Bladbjerg EM, Bor MV, et al. SAMeTT2R2 does not predict time in therapeutic range of the international normalized ratio in patients attending a high-quality anticoagulation clinic. Chest. 2014;145:188–189. doi: 10.1378/chest.13-1897. [DOI] [PubMed] [Google Scholar]
- 35.Palareti G, Antonucci E, Lip GY, et al. The SAMe-TT2R2 score predicts the quality of anticoagulation control in patients with acute VTE. A real-life inception cohort study. Thromb Haemost. 2016;115:1101–1108. doi: 10.1160/TH15-10-0830. [DOI] [PubMed] [Google Scholar]
- 36.Abumuaileq RR, Abu-Assi E, Raposeiras-Roubin S, et al. Evaluation of the SAMe-TT2R2 risk score for predicting the quality of anticoagulation control in a real-world cohort of patients with non-valvular atrial fibrillation on vitamin K antagonists. Eurospace. 2015;17:711–717. doi: 10.1093/europace/euu353. [DOI] [PubMed] [Google Scholar]
- 37.Macedo AF, Bell J, McCarron C, et al. Determinants of oral anticoagulation control in new warfarin patients: Analysis using data from Clinical Practice Research Datalink. Thromb Res. 2015;136:250–260. doi: 10.1016/j.thromres.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Rose AJ, Hylek EM, Ozonoff A, et al. Patient characteristics associated with oral anticoagulation control: Results from the Veterans AffaiRs study to Improve Anticoagulation (VARIA) J Thromb Haemost. 2010;8:2182–2191. doi: 10.1111/j.1538-7836.2010.03996.x. [DOI] [PubMed] [Google Scholar]
- 39.Chew TW, Gau CS, Wen YW, et al. Epidemiology, clinical profile and treatment patterns of venous thromboembolism in cancer patients in Taiwan: A population-based study. BMC Cancer. 2015;298:1–10. doi: 10.1186/s12885-015-1200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dlott JS, George RA, Huang X, et al. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014;129:1407–1414. doi: 10.1161/CIRCULATIONAHA.113.002601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.