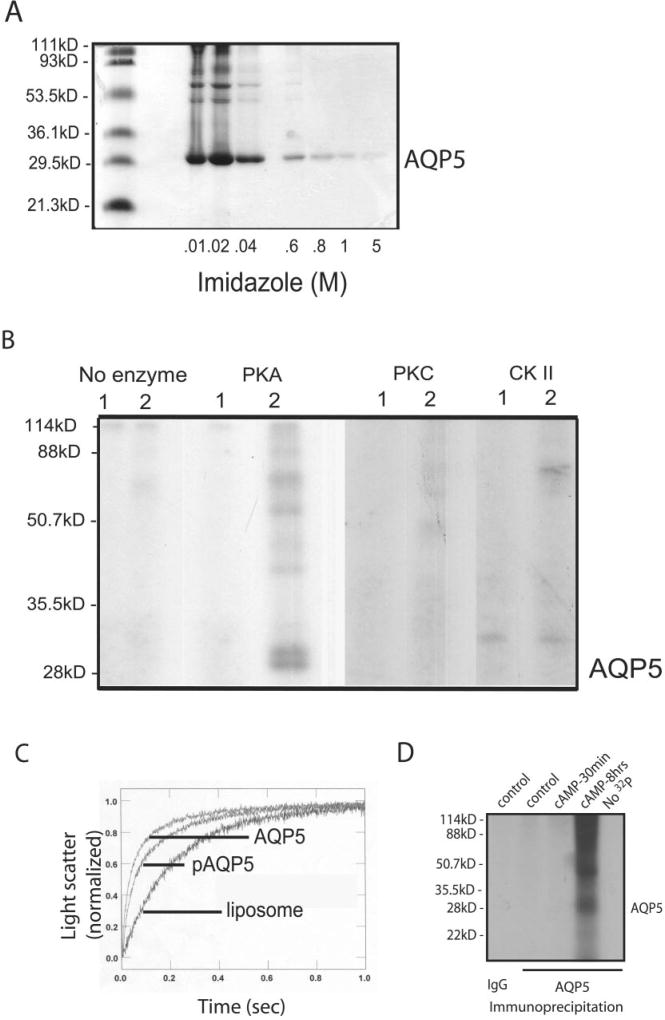
Fig. 9. Phosphorylation of AQP5.
A, Coomassie showing purified AQP5 eluted by imidazole. B, purified AQP5 protein was reconstituted into liposomes and incubated with [γ-32P]ATP in the absence or presence of PKA, PKC, or CKII; (1) control liposomes; (2) AQP5-containing proteoliposomes. Samples were resolved by gel electrophoresis and exposed to film to assess phosphorylation. Only PKA produced AQP5 phosphorylation; higher MW bands consistent with oligomers of AQP5 can be seen. Labeling observed in the CKII lanes is at a higher MW than AQP5 and is consistent with autophosphorylation of CKII (50). C, control and AQP5-containing proteoliposomes were examined for water permeability by stopped flow. AQP5 was highly water permeable compared with control liposomes. Water permeability of phosphorylated AQP5 was not statistically different from nonphosphorylated AQP5. D, MLE-12 cells were incubated with 32P for 30 min, exposed to control media or media containing pCPT-cAMP (100 µm), and harvested at the specified times. Control immunoprecipitates (IgG) or anti-AQP5 immunoprecipitates were resolved by gel electrophoresis and exposed to film for assessment of phosphorylated proteins.