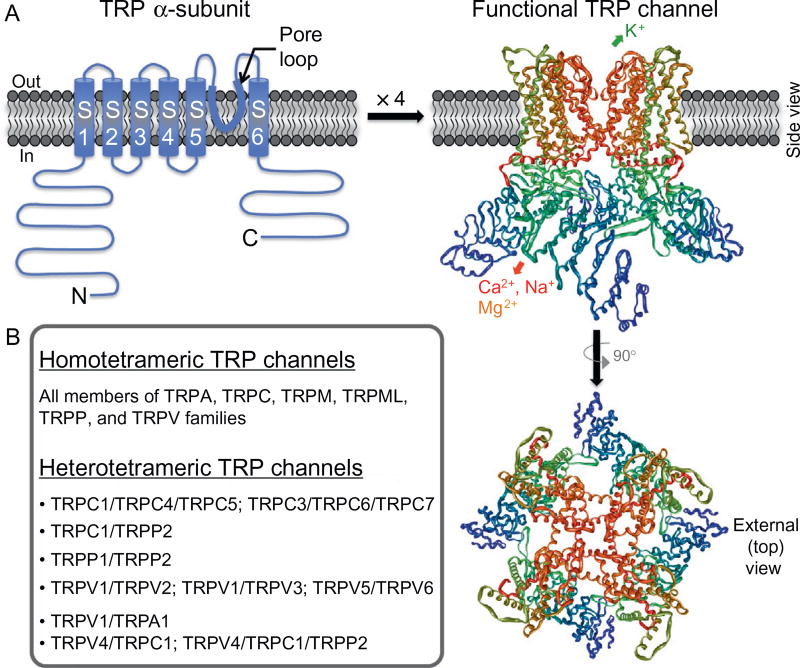
Figure 2.
Structural features of functional TRP channels. (A) Model of predicted secondary structure topology of a monomeric TRP channel subunit (called α-subunits) on a plasma membrane lipid bilayer. S1–S6 denote transmembrane segments/domains 1 through 6, with a hydrophilic pore loop between S5 and S6 transmembrane segments, and N and C denote cytoplasmic amino- and carboxy-terminals, respectively. Four monomeric α-subunits assemble in a three-dimensional arrangement to make a functional channel, which is shown on the right. The structure was constructed from the PDB structure file obtained from the protein data bank, originally submitted by Refs. 8,9 from their cryoelectron microscopic structure of TRPV1. (B) List of homo- and heterotetrameric TRP channels formed within individual TRP subfamily member channels, as well as in combination of inter-subfamily TRP channel members.