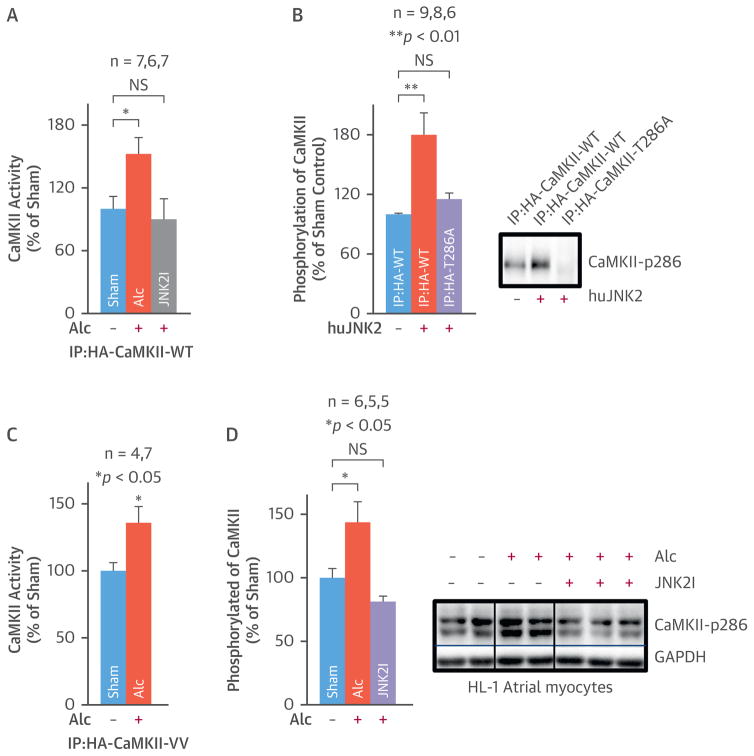
FIGURE 7. Alcohol-Evoked JNK2 Regulates CaMKII Activity.
(A) Summarized data show increased ADP production (reflecting an increased ATP consumption in the CaMKII reaction with its substrate) in HA-pulldown CaMKII-WT proteins from alcohol-exposed cells, while JNK2I suppressed alcohol-evoked CaMKII activity. (B) Pooled ADP production assay data and representative immunoblotting images show that active pure JNK2 proteins increase the phosphorylation of CaMKII-WT, whereas autophosphorylation site mutation prevents this JNK2 action on HA-pulldown CaMKII-T286A proteins. (C) Summarized data show alcohol-enhanced CaMKII activity is not affected by the mutation of CaMKII oxidative sites Met280/281. (D) Summarized quantitative immunoblotting data and representative images show JNK2 inhibition prevented alcohol-enhanced phosphorylation of CaMKII (activated) in cultured HL-1 myocytes. CaMKII = calmodulin kinase; CaMKII-WT = wildtype CaMKII; CaMKII-T286A = CaMKII with Thr287Ala mutation; CaMKII-VV = CaMKII with Met280/281Val mutation; JNK2I = JNK2 inhibitor. Other abbreviations as in Figures 1 and 2.