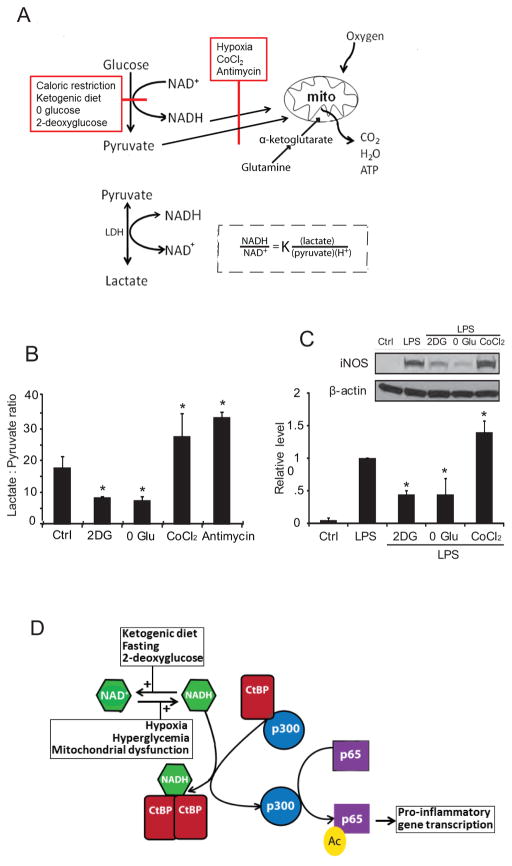
Figure 5. NADH and CtBP mediate coupling between glucose metabolism and gene transcription.
(A) Factors that slow glucose flux through glycolysis, such as reduced glucose availability or glycolytic inhibitors, reduce NADH levels and thereby reduce NADH:NAD+ ratio, whereas factors that slow oxidative metabolism, such as hypoxia and mitochondrial inhibitors, have the opposite effect. Lactate dehydrogenase (LDH) maintains the lactate:pyruvate ratio in equilibrium with the cytosolic NADH:NAD+ ratio. In the experiments shown, glutamine provided α-ketoglutarate to fuel mitochondrial ATP production in the absence of glycolysis. (B) The lactate:pyruvate ratio provides an index of the cytosolic NADH:NAD+ ratio in cells treated with glycolytic inhibition (2DG, 1 mM 2-deoxyglucose; 0 Glu, glucose-free medium) or mitochondrial inhibitors (CoCl2, 200 μM cobalt chloride; antimycin, 1 μM antimycin A). n = 4; *p < 0.05 v. control. (C) LPS-induced iNOS expression was suppressed in RAW267.4 cells treated with 1 mM 2DG or glucose-free medium, and was increased in cells treated with the CoCl2. n = 4; * p < 0.05 v. control. Error bars show s.e.m. (D) CtBP normally binds and inhibits p300 acetylase. NADH promotes CtBP dimerization and resultant release of p300 from CtBP. Unbound p300 acetylates the p65 subunit of NF-κB to promote NF-κB transcriptional activity. Fasting, ketogenic diet, and glycolytic inhibition reduce NADH levels and thereby reduce CtBP dimerization and increase CtBP repression of p300 activity. Hypoxia, hyperglycemia, and mitochondrial dysfunction have the reverse effect. Panels A, B, and C are from (Shen et al. 2017).