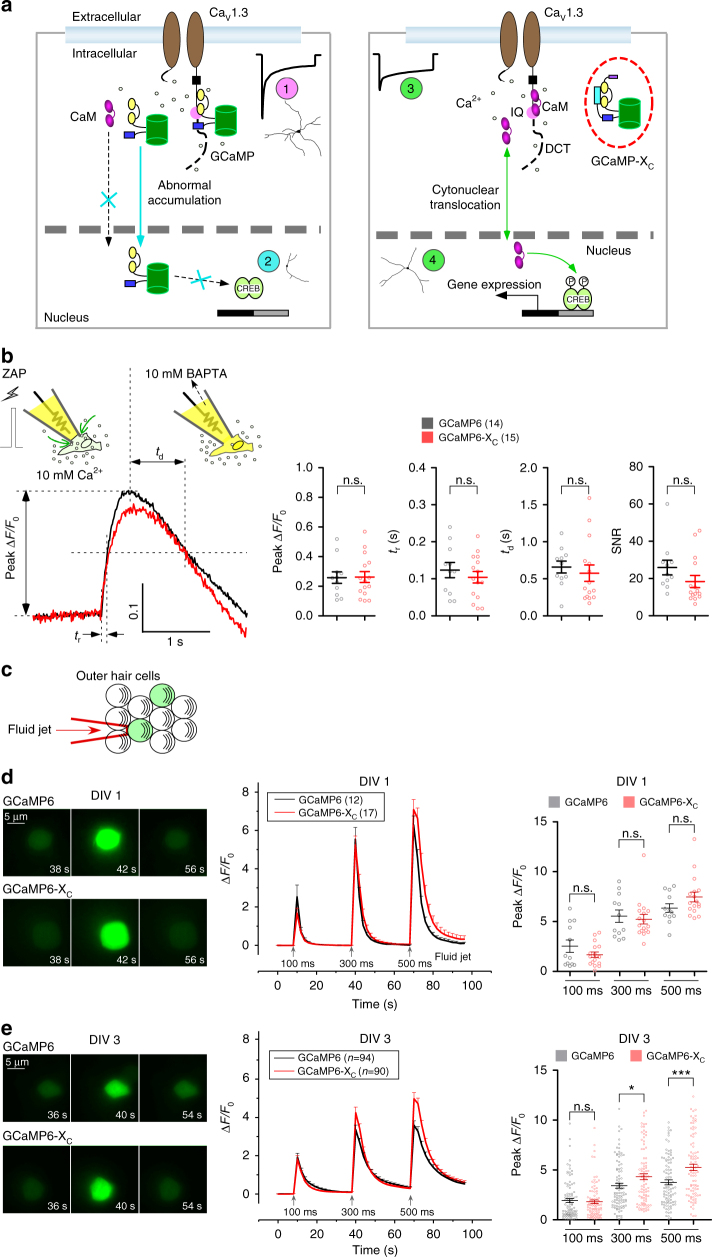
Fig. 6.
Schematic summary and sensor performance for GCaMP and GCaMP-X. a GCaMP defects and advantages of GCaMP-X. In neurons, GCaMP sensors behave as CaM-like proteins perturbing CaV1 gating and distorting Ca2+ dynamics (1). The abnormal enhancement of CaV1 by cytosolic GCaMP underlies overgrown neurites. Different from endogenous CaM, GCaMP itself could no longer properly translocate into the nucleus in response to membrane excitation, which also dominant-negatively impairs the acute mobility of endogenous CaM. Once present in the nucleus (by accumulation or NLS), nuclear GCaMP perturbs transcription signaling and gene expression potentially through its aberrant CaM motif, impairing general health of neurons including Ca2+ signals (reflected as abnormal sensor readouts) and neurite outgrowth (2). The new GCaMP-X sensors are designed with apoCaM protection and explicit localization (e.g., GCaMP-XC for cytosolic Ca2+) to eliminate GCaMP perturbations on CaV1 gating (3) and signaling (4). b Sensor performance of GCaMP-XC validated by single-AP like Ca2+ transients in HEK293 cells. Briefly, glass electrodes formed the Giga-Ohm seal with cell membrane (resting); then 3 ms ZAP for break-in generated a rapid Ca2+ influx due to transient membrane rupture, immediately followed by a fast decay arising from strong Ca2+ chelators of 10 mM BAPTA included in the pipette solution. Key indices of peak ΔF/F0, rise time tr, decay time td and SNR were compared between GCaMP6m and GCaMP6m-XC. Epi-fluorescence images were acquired at the sampling rate of 100 Hz or higher. c–e Sensor performance of GCaMP-XC validated with mechanosensing outer hair cells. Cells were transfected with GCaMP6m or GCaMP6m-XC at P1 by electroporation, and cultured for 1 day (DIV 1) or 3 days (DIV 3). Fluid-jet pulses of 100, 300 or 500 ms were applied to cells (c). Representative images, normalized fluorescence changes (ΔF/F0) indicative of Ca2+ dynamics and statistical summary of peak ΔF/F0 were compared between DIV 1 (d) and DIV 3 (e) cells expressing GCaMP6m or GCaM6m-XC. Standard error of the mean (S.E.M.) and Student’s t-test (two-tailed unpaired with criteria of significance: *p < 0.05; **p < 0.01, and ***p < 0.001) were calculated when applicable, and n.s. denotes “not significant”