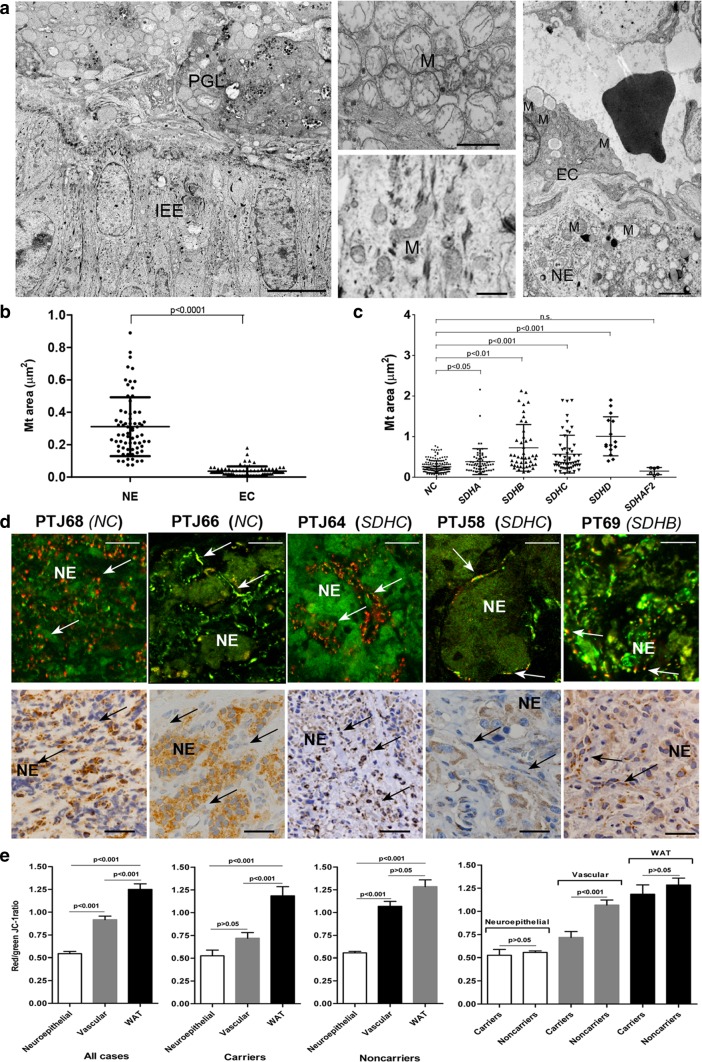
Fig. 2.
Morphofunctional alterations of mitochondria in the neuroepithelial component of paraganglioma. a The transmission electron micrograph on the left shows the interface between normal inner ear epithelium (IEE, bottom half of the microscopic field) and an underlying non-embolized tympanic area of a tympano-jugular paraganglioma (PTJ64, from an SDHC mutation carrier, top half). The central higher magnification views illustrate the ultrastructure of the mitochondria in IEE cells (bottom) and in neuroepithelial paraganglioma cells (top). Mitochondrial hyperplasia with swelling and consequent loss of cristae is clearly evident in paraganglioma cells, while the mitochondria of the adjacent inner ear epithelial cells are structurally normal, which rules out the possibility that the mitochondrial alterations might have been due to surgical/post-surgical manipulation. The transmission electron micrograph on the right, focused on another PTJ64 paraganglioma field, illustrates the morphological differences between the roundish, swollen mitochondria with disrupted cristae routinely found in neuroepithelial cells (NE) and the smaller, relatively well-preserved mitochondria found in the adjacent endothelial cells (EC). The capillary lumen contains an erythrocyte. Bars = 10 µm (left image) and 1 µm (centre and right images). b Scatter plots of mitochondrial cross-sectional areas (µm2) determined on electron micrographs of neuroepithelial (NE) and endothelial cells (EC) from 20 paragangliomas (69 measurements randomly extracted from a total of 354 measurements available for NE cells compared to 69 measurements for endothelial cells). c Scatter plots of mitochondrial cross-sectional areas (µm2) determined on electron micrographs of neuroepithelial (NE) paraganglioma cells presented according to germline SDHx status. Mean areas were: 0.258 ± 0.150 µm2 (noncarriers, NC, 168 measurements); 0.384 ± 0.318 µm2 (mutated in SDHA, 66 measurements, P < 0.05); 0.724 ± 0.573 µm2 (mutated in SDHB, 45 measurements, P < 0.01); 0.568 ± 0.465 µm2 (mutated in SDHC, 54 measurements, P < 0.001); 1.008 ± 0.480 µm2 (mutated in SDHD, 15 measurements, P < 0.001); 0.152 ± 0.087 µm2 (mutated in SDHAF2, 6 measurements, P > 0.05). P-values were calculated using one-way anova with Games-Howell post hoc test. d JC-1 fluorescence highlights collapse of the mitochondrial membrane potential in the neuroepithelial component. A set of 5 paragangliomas is shown, two from SDHx noncarriers (PTJ68, PTJ66) and three positive for germline mutation in SDHC (PTJ64 and PTJ58) and SDHB (PT69). Regions of high mt polarization (top panels, red to yellow fluorescence, indicative of concentration-dependent J-aggregates, arrows) largely correspond to the vascular/glial component, while the neuroepithelial nests and cords (NE) are depolarized, as indicated by the green fluorescence due to JC-1 monomers. Micrographs in the panels below show SDHB immunohistochemistry for the same tumors, where arrows point to the vascular/perivascular component that supports more or less defined neuroepithelial zellballens (ZB). Brown granular SDHB staining is undetectable in the neuroepithelial components of PT69, whereas PTJ58, PTJ64, PTJ66, PTJ68 are weakly to strongly positive. The variability in SDHB protein immunohistochemistry contrasts with the constant evidence of mt depolarization in the neuroepithelial component, which is unrelated to SDHx/SDHB status. Bars = 20 µm. Hematoxylin is used as a counterstain in SDHB immunohistochemistry. e The red/green JC-1 fluorescence ratios, indicative of mt membrane potential, decrease in the vascular (mean: 0.92 ± 0.04, all cases; 0.72 ± 0.06, carriers; 1.07 ± 0.05, noncarriers) and, more prominently, neuroepithelial (mean: 0.54 ± 0.02, all cases; 0.53 ± 0.06, carriers; 0.56 ± 0.02, noncarriers) paraganglioma components compared to the autologous white abdominal adipose tissue (WAT, mean: 1.25 ± 0.06, all cases; 1.18 ± 0.1, carriers; 1.28 ± 0.07, noncarriers). The differences between the neuroepithelial and the vascular tumour components and between the neuroepithelial tumour component and WAT are significant. Data are from 11 PGLs, of which 4 associated with identified germline SDHx mutations and 7 SDHx mutation-negative