Abstract
Despite more than a century of biological research on the evolution and maintenance of mimetic signals, the relative frequencies of models and mimics necessary to establish and maintain Batesian mimicry in natural populations remain understudied. Here we investigate the frequency-dependent dynamics of imperfect Batesian mimicry, using predation experiments involving artificial butterfly models. We use two geographically distinct populations of Adelpha butterflies that vary in their relative frequencies of a putatively defended model (Adelpha iphiclus) and Batesian mimic (Adelpha serpa). We found that in Costa Rica, where both species share similar abundances, Batesian mimicry breaks down, and predators more readily attack artificial butterfly models of the presumed mimic, A. serpa. By contrast, in Ecuador, where A. iphiclus (model) is significantly more abundant than A. serpa (mimic), both species are equally protected from predation. Our results provide compelling experimental evidence that imperfect Batesian mimicry is frequency-dependent on the relative abundance of models and mimics in natural populations, and contribute to the growing body of evidence that complex dynamics, such as seasonality or the availability of alternative prey, influence the evolution of mimetic traits.
Keywords: Batesian mimicry, frequency dependence, predation, abundance, seasonality
1. Introduction
Batesian mimicry occurs when a palatable or undefended species (the ‘mimic’) resembles an unpalatable or otherwise defended species (the ‘model’), and thereby gains protection from predators [1]. For over a century ecologists and evolutionary biologists have studied the dynamics of this phenomenon, with much attention paid to the evolution and maintenance of mimetic signals [2–4]. Although degree of capture cost and predator learning play substantial roles in mimicry system effectiveness [4], arguably the most critical components to successful Batesian mimicry in a population are the relative frequency and abundance of models and mimics, and phenotypic similarity of the mimic to the model.
It has been widely predicted that predator attack rates on Batesian mimics should depend not only on the general presence of the defended model, but also on the relative (and absolute) abundances of mimics and models [4–8]. Brower's [9] classic in-cage experiment showed that avian predation on palatable mimetic prey (mealworms) increased as the percentage of unpalatable models available decreased, and a similar study with toad and frog predators consuming honeybee prey resulted in a decrease in mimic predation as the presence of the model increased [10]. A more recent field study by Pfennig et al. [11] found that kingsnake replicas experience greater carnivore attacks in geographical locations where their model, the coral snake, is absent, and this result is consistent across gradients ranging from sympatry to allopatry. This supports the view that mimics receive more protective benefits when they are sympatric with models, hence with protection dependent upon the physical presence of the model. Similarly, successful Batesian mimicry is also contingent upon how closely or perfectly mimics resemble models when model abundance is variable [12–14]. However, very little experimental work in natural field settings has been undertaken to examine the effects of imperfect Batesian mimic and model abundances on local predator behaviour, especially with two parallel populations that vary in relative model : mimic frequencies.
Butterflies have long served as textbook examples of Batesian mimicry [1,15–18], and recent work has shown that imprecise mimetic colour patterns in tropical Adelpha butterflies may result in the ability for predators to learn—and attack—palatable mimics in a given population [19]. Convergent evolution in Adelpha is widespread, and many species (such as Adelpha iphiclus and Adelpha serpa) share strikingly similar colour pattern phenotypes in sympatry [20–22]. Similar patterns in unrelated species suggest that convergence is driven by selection rather than shared ancestry (electronic supplementary material, figure S1). Adelpha iphiclus caterpillars feed almost exclusively on Rubiaceae hosts, which are speculated to be toxic to most herbivores based on the alkaloids known to be present in many Rubiaceae species [23–25]. On the other hand, A. serpa caterpillars are generalists on primarily non-toxic hosts [19], and it has been hypothesized that mimicry occurs in Adelpha between such unpalatable Rubiaceae-feeding specialists and palatable non-Rubiaceae-feeding generalists [19,26].
Finkbeiner et al. [19] examined predation rates on the presumed unpalatable Rubiaceae-feeding model, A. iphiclus, and its presumed non-Rubiaceae-feeding palatable mimic, A. serpa. Although both species appear nearly identical, they bear slight differences in the white spectral reflectance on the dorsal wing (electronic supplementary material, figure S2 and table S1). Avian predators readily distinguished between paper models of the two species in a Costa Rican field location, preferring to attack mimic A. serpa, whereas model A. iphiclus was avoided (as predicted if A. iphiclus are indeed unpalatable) [19]. This result, indicating that Batesian mimicry was not effective, suggested several alternative explanations for convergent wing patterns in these butterflies, including: (i) historical shifts in palatability and host plant use from a formerly Müllerian mimetic system (where both model and mimic bear a cost), (ii) spatial/temporal variation in Adelpha toxicity, (iii) anti-predatory benefits via disruptive colouration, (iv) thermoregulatory advantages, (v) developmental constraints generating convergent expression patterns or (vi) sexual selection. Although each of these mechanisms is plausible, the strong results from Finkbeiner et al. [19], and extensive preliminary data about relative model : mimic frequencies in the field, suggested that geographical variation in frequency-dependent Batesian mimicry dynamics [27,28] was the most likely explanation for these paradoxical results.
To test frequency-dependent selection in mimetic Adelpha, and examine the efficacy of imprecise Batesian mimicry, we replicated our predation study using artificial butterfly prey at a second, independent field site in eastern Ecuador. As outlined below, abundance data for this location indicate that the putative, palatable mimic (A. serpa) is much less abundant relative to the presumed model (A. iphiclus) in comparison with our original field site in Costa Rica. Therefore, we hypothesized that the uncommon mimic A. serpa would be protected by the presence of common model A. iphiclus in Ecuador, in contrast to higher predation on the mimic in Costa Rica where the model is less common. Our results provide compelling experimental field evidence that imperfect Batesian mimicry is frequency-dependent upon the relative abundance of models and mimics in natural populations, and contribute to the growing body of evidence that complex dynamics underlie the evolution of mimetic traits.
2. Material and methods
(a). Field sites
All data collection was completed in Costa Rica and Ecuador. Field sites were chosen based on the known presence of Adelpha butterflies and active avian predators. In Costa Rica, we used the Organization for Tropical Studies' (OTS) La Selva Biological Reserve in Sarapiquí (10°25′28″ N; 84°0′18″ W). This reserve is composed of both primary- and secondary-growth Caribbean lowland forest, with patches of abandoned plantations and open pasture. Our field location in Ecuador was the Yasuní Research Station (0°40′27″ S, 76°23′50″W) operated by the Pontifica Universidad Católica del Ecuador (PUCE). This site principally consists of primary lowland Amazon rainforest, although patches of disturbed areas surround the reserve. Predation experiments in Ecuador were carried out during January–February 2017 at the end of the dry season, and butterfly abundance data were collected during August 2014–April 2017. A previously executed predation study in Costa Rica [19] occurred during March–April 2016, also during the end of the dry season (consistent with the same season for—and within a year of—the Ecuador predation study), and abundance data in Costa Rica were collected during March 2015–August 2016.
(b). Production of artificial butterflies
To measure predator responses to different butterfly phenotypes, we used artificial butterfly models in predation studies. This method has been successfully demonstrated in recent studies with butterfly prey [19,29–35], and allows us to generate large sample sizes of thousands of individuals. Artificial butterflies were designed and constructed following protocols in Finkbeiner et al. [19,33–35], where visually accurate paper wings were created by referencing spectral properties in natural butterfly wings, taken from specimens in both Ecuador and Costa Rica. Spectral reflectance measurements were taken using an Ocean Optics USB2000 fibre-optic spectrometer, with a bifurcating cable (R400-7-UV-vis Ocean Optics, Winter Park, FL) connected to a deuterium–halogen tungsten light source (Model MINIDT1000-027; Analytical Instrument Systems, Flemington, NJ). The detecting fibre was fixed at an angle of 45° to the plane of the butterfly wing, and a white spectralon standard (WS-1-SL; Labsphere, North Sutton, NH) was used during calibration. A new white standard was taken approximately every five measurements to confirm spectral consistency. Artificial butterfly models were designed showing the dorsal side of the butterfly wings, as if butterflies were resting/basking on leaftops (figure 1).
Figure 1.
Artificial butterfly models representing (a) A. iphiclus, the presumed toxic model, and (b) A. serpa, the putative non-toxic Batesian mimic. Models on the right-hand side show evidence of avian attack, indicated by beak imprints on the plasticine abdomen.
The quantum catches for stimuli [36] were calculated, and discriminability between artificial models and natural wing reflectance spectra (for both Costa Rica and Ecuador specimens and models) was determined using tetrachromatic bird-vision models from Vorobyev & Osorio [37]. The comparisons were made using the blue tit (Cyanistes caeruleus, UV-type), and chicken (Gallus gallus, violet-type) cone sensitivities (electronic supplementary material, table S1). For chicken, we used the behaviourally determined parameters of Olsson et al. [38], namely, a Weber fraction = 0.06 and relative abundances of cones (VS = 0.25, S = 0.5, M = 1, L = 1). For the blue tit, we followed the work of Hart et al. [39] and used a Weber fraction = 0.05 and relative abundances of cones (UV = 0.37, S = 0.7, M = 0.99, L = 1). High light intensity and Endler's daylight or forest shade irradiance spectra [40] were used. Comparisons were made for white reflectance spectra only, because white is considered an important mimetic feature in other butterfly mimicry systems [41,42], and visual inspection of the reflectance spectra did not reveal any differences between the other wing colours, orange and brown (electronic supplementary material, figures S3–S4).
Butterfly models were printed using an Epson Stylus Pro 4900 printer with UltraChrome HDR ink on Whatman qualitative filter paper (no. 1001-917). Filter paper has reflective-neutral properties, providing reflectance spectra brightness similar to brightness in actual butterfly wings [33]. Because not all reflectance properties were easily reproduced with printed colours, we enhanced some colours on artificial butterfly wings using Crayola crayons (Easton, PA) to achieve a better spectral match. Sheets of printed models were pasted onto cardstock backings with spray adhesive (Krylon Products Group, Cleveland, OH) and cut using a Brother ScanNCut machine (Brother International, Bridgewater, NJ). To provide additional durability, edges of model wings were dipped in wax, and then twist-ties were threaded through the model centre before plasticine ‘abdomens’ (Newplast) were added to allow detection of avian predator attacks.
(c). Mimicry predation experiments
Field predation studies were carried out to determine predation rates on two common mimetic species in Costa Rica and Ecuador: A. iphiclus (model) and A. serpa (Batesian mimic). Both species are found locally in the Costa Rica and Ecuador sites, but vary in their abundances and presumed toxicity, with A. iphiclus presumed to be the unpalatable model and A. serpa the palatable mimic. Predation was also measured on two other butterfly species during the same study, palatable Junonia evarete and novel (non-indigenous) Adelpha leucophthalma. Although we report results for all four model phenotypes used, our principle focus is on predation in A. iphiclus (model) and A. serpa (mimic) (see [19] for information on Junonia and A. leucophthalma artificial butterfly models). We constructed 500 individuals of each phenotype for each field site, totalling 1000 butterflies for the iphiclus/serpa comparison or 2000 butterflies for the overall comparisons with the four phenotypes (as in [19]). In both Costa Rica and Ecuador, 100 forest sites were selected and separated approximately 150–250 m apart to control for avian predator home range overlap, allowing us to sample a broad avian population in each reserve. In each forest site, five of each of the butterfly phenotypes were set in alternating order and at least 4 m apart, and approximately 1 m above the ground on appropriate foliage to resemble butterflies at rest. Butterfly models were attached onto twigs and others onto leaves, and the model phenotypes placed on twigs versus leaves were randomized.
Artificial butterfly models were exposed to predators for four days (96 h), checked daily for avian predator attacks, and then removed. If a model was attacked more than once during the four-day experimental period, or if more than one apparent attack mark was observed on the model, we counted this as only one attack in the analysis. Attacked models were replaced daily. Avian attacks were confirmed by the presence of beak imprints on the plasticine abdomen (figure 1; examples in [19,33–35]). Previous studies have used a four-day predator exposure time for artificial butterfly prey in the tropics [19,29–35], suggesting that this amount of time is enough for predators to come in contact with (and attack) the models. Any artificial butterfly models left exposed for longer periods of time risk being learned as false prey, which may affect predator behaviour and therefore skew prospects for long-term survival data; nevertheless, longer-term survival projects on butterfly mimicry systems have proved to be useful in some studies [43,44]. Butterfly model survivorship across the four-day experimental period was analysed using a Cox proportional-hazards regression model (‘survival’ package in R [45]) with post hoc Wald tests between phenotype pairs [31,46].
(d). Local abundance data
Butterfly abundance data for A. iphiclus and A. serpa were collected in both our Costa Rica and Ecuador field studies prior to, during and after predation experiments. Records for both species in Costa Rica were obtained monthly from March 2015 to August 2016 (18 months), using a combination of trapping, netting, sighting/observation and larval collection on hosts. Trapping studies included checking 64 traps for five consecutive days during the first week of each month (32 fruit bait traps, 32 carrion bait traps, in both canopy and understorey); trap sites were separated by a minimum of 50 m, with understorey traps placed approximately 1.25–1.5 m above the ground and canopy traps at an average height of 22.1 ± 5.4 m. Netting consisted of capturing, marking and identifying adult butterflies; sightings were noted observations of marked or unmarked individuals at perch sites; and species were identified with binoculars based on forewing orange patch characteristics and white band hue, which, in A. serpa (mimic), appears more blue-green to the human eye than A. iphiclus (model) (see electronic supplementary material, figure S2 for quantitative support). Collected larvae were reared to adults and species confirmed upon eclosure. Ultimately, we excluded information regarding larval records because this was not directly indicative of adult abundance.
Data on abundance of A. iphiclus and A. serpa in Ecuador were recorded monthly from August 2014 to April 2017. Ecuador trapping methods were identical to Costa Rica, with a total of 64 traps at the field location (32 fruit bait, 32 carrion bait, divided into both understorey and canopy at 0.7 ± 0.13 m and 17.8 ± 4 m high, respectively). We report only trapping results for the 22 months surrounding the experimental predation study (electronic supplementary material, table S2b). Hand-collected specimens were also documented in this same field site, but well before the predation study during July–October 2015. Although trapping studies overlapped temporally with predation experiments, we set artificial butterfly models in different locations of the reserve as the traps to avoid any disruption the traps may have on the local adult butterfly and bird communities during predation studies. To compare reported abundances between A. serpa and A. iphiclus in Costa Rica, as well as separately in Ecuador, we performed a two-tailed Wilcoxon signed-rank test with continuity correction in R [45] for each pair.
3. Results
(a). Predation on locally convergent phenotypes
Our initial predation experiment in Costa Rica [19] tested rates of avian predator attack on four different butterfly phenotypes, with attention paid to attacks on presumed unpalatable A. iphiclus (model) and convergent presumed palatable A. serpa (mimic). In total, 189 combined avian attacks were recorded on the 2000 total models (500 of each phenotype), among the 100 selected forest sites (table 1). We recorded 30 attacks on A. iphiclus (6.0% attacked) and 56 attacks on A. serpa (11.2% attacked), as well as 52 attacks on Junonia (10.4% attacked) and 51 on A. leucophthalma (10.2% attacked), whose comparisons are described and discussed in detail in [19]. Survivorship comparisons between model A. iphiclus and mimic A. serpa showed statistically significant differences in survivorship between the two phenotypes (while implementing all four experimental phenotypes in initial multiple-comparison tests), where A. iphiclus had higher survivorship than A. serpa (Cox proportional-hazards regression model, Wald = 5.56, d.f. = 1, p = 0.0184; figure 2a).
Table 1.
Daily number of attacks for each butterfly model phenotype.
| A. iphiclus (model) | A. serpa (mimic) | Junonia (control) | A. leucophthalma (novel) | |
|---|---|---|---|---|
| total attacks (Costa Rica) | 30a | 56 | 52 | 51 |
| total attacks (Ecuador) | 10 | 9 | 17 | 20 |
aAdelpha iphiclus (model) has a statistically significantly higher (p > 0.05) survival rate than A. serpa (mimic).
Figure 2.
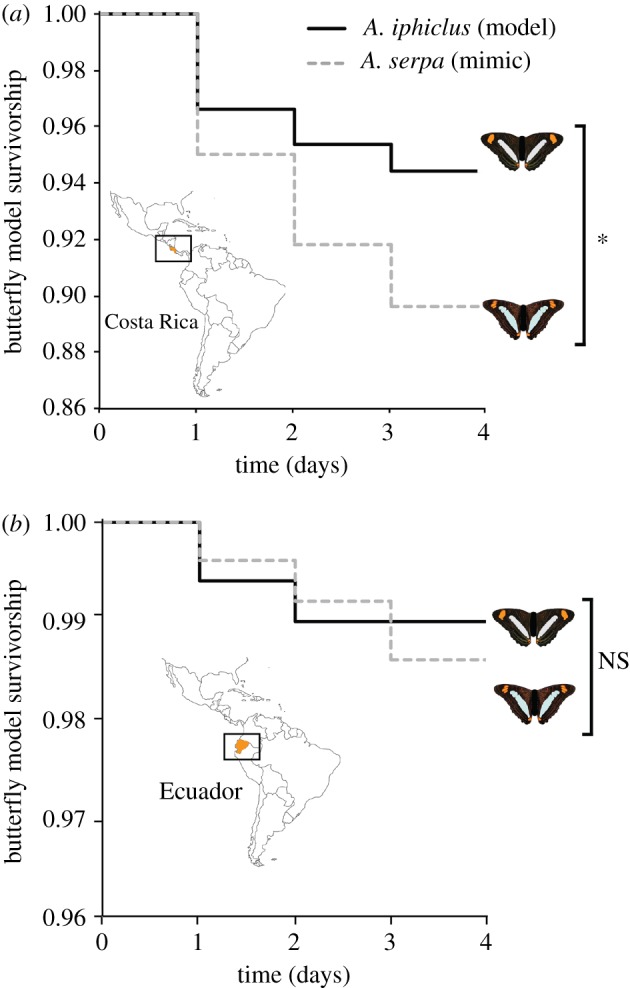
Artificial butterfly models experience different survivorship during predation field studies in (a) Costa Rica and (b) Ecuador. Shown are survivorship curves (Cox proportional-hazards regression model) where A. iphiclus (model) has a significantly higher survivorship than A. serpa (mimic) in Costa Rica (*p = 0.0184), and both species have similar survivorship in Ecuador (NS, p = 0.801).
The parallel study in Ecuador recorded a total of 56 combined avian attacks across all four model phenotypes, with 10 attacks on model A. iphiclus (2.0% attacked), 9 attacks on mimic A. serpa (1.8% attacked), 17 attacks on Junonia (3.4% attacked) and 20 attacks on A. leucophthalma (4.0% attacked; table 1). Results showed non-significant differences in survivorship between A. iphiclus and A. serpa (Cox proportional-hazards regression model, Wald = 0.06, d.f. = 1, p = 0.801; figure 2b), suggesting that they are attacked at equal rates by avian predators. In general, the proportion of attacks on A. iphiclus (model) relative to all other models at both sites is similar (18% Ecuador versus 16% Costa Rica), whereas A. serpa (mimic) is attacked at nearly twice the rate of A. iphiclus (16% versus 30%) in Costa Rica but is treated virtually identically by avian predators in Ecuador. Although low statistical power could be responsible for non-significance in attack rates between A. iphiclus and A. serpa in Ecuador, similarities in the proportion of attacks for model A. iphiclus and palatable control Junonia in both field locations lead us to conclude that statistical power does not influence the outcome of our results. Note that here we limit our presentation about rates of predation on the control (Junonia) and novel (A. leucophthalma) species, when present at equal abundances to the model A. iphiclus and mimic A. serpa, because they have previously been discussed in [19].
(b). Variation in butterfly abundances between field locations
Adult A. iphiclus and A. serpa abundance records at both field locations are presented in table 2, and month-to-month records are presented in electronic supplementary material, table S2. In Costa Rica during March 2015–August 2016, 18 A. iphiclus (model) and 22 A. serpa (mimic) were reported from adult sightings and netting. Including trapping data, this total increased to 36 A. iphiclus and 22 A. serpa; no A. serpa individuals were collected in traps at this field site. No marked individuals were recaptured. Results from our Wilcoxon signed-rank test, including both trapping and non-trapping data, showed no overall differences in the abundances of putative unpalatable model A. iphiclus and putative palatable mimic A. serpa across the sampling period (Wilcoxon signed-rank test, V = 46.5, d.f. = 34, p = 0.582). During the specific time period that our predation experiment was completed in Costa Rica (March–April 2016), there were three total adult records of A. serpa but no adult records of A. iphiclus, and in the months leading to this experiment (January–March 2016) only one adult A. iphiclus was recorded, whereas a total of 12 A. serpa adults were recorded (including the three individuals noted in March 2016). Records for May through August showed a spike in A. iphiclus (model) abundance (electronic supplementary material, table S2a), but during our experimental period A. serpa (mimic) had a higher abundance in this field location.
Table 2.
Observed adult abundance records of A. iphiclus (model) and A. serpa (mimic) at two field locations: Costa Rica (18-month records) and Ecuador (22-month records). For month-to-month abundance records, see electronic supplementary material, table S2.
| collected/sighted A. iphiclus | collected/sighted A. serpa | trapped A. iphiclus | trapped A. serpa | total A. iphiclus | total A. serpa | |
|---|---|---|---|---|---|---|
| Costa Rica | 18 | 22 | 18 | 0 | 36 | 22 |
| Ecuador | 15 | 0 | 69 | 1 | 84a | 1 |
aAdelpha iphiclus is significantly more abundant (p > 0.05) than A. serpa.
In Ecuador, we recorded 69 adult A. iphiclus (model) and only one adult A. serpa (mimic) from trapping studies during July 2015–April 2017 (five of these 69 A. iphiclus reports were recaptures). Hand-collected records (netting) during July–October 2015 report 15 A. iphiclus (one recapture), while no A. serpa were collected. Combining both trapping and netting records, we found a strong statistical difference between the abundances of A. iphiclus and A. serpa at the Ecuador study site (Wilcoxon signed-rank test, V = 136.0, d.f. = 42, p = 0.00047; electronic supplementary material, table S2b) where A. iphiclus (model) is highly abundant and A. serpa (mimic) is not. During the predation experiments (January–February 2017), five adult A. iphiclus, but no A. serpa, were recorded. Trapping data suggest A. iphiclus were much more abundant during the six months preceding our predation experiment, indicating possible seasonality and implying that avian predators probably encountered the model prior to our predation experiments.
4. Discussion
Here we assessed survivorship on two phenotypically convergent butterfly species with slightly imperfect mimetic colour patterns, and with presumed differences in palatability (one toxic and the other non-toxic), in two different geographical locations with variable species abundance. We found that in Costa Rica, where the local abundances of A. iphiclus (toxic model) and A. serpa (non-toxic mimic) are similar, avian predators avoided A. iphiclus and attacked A. serpa. However, in Ecuador, where the putative mimic A. serpa is significantly less abundant than A. iphiclus, both species were equally avoided. Contrary to results in the Costa Rica location [19], and in support of our hypothesis for frequency-dependent mimicry, the results from Ecuador indicate that A. serpa gains protection from predators by sharing a convergent phenotype with the highly abundant, and presumably toxic model, A. iphiclus. We interpret this result as evidence for geographical variation in the frequency-dependent dynamics of imperfect mimicry.
Indeed, our abundance data show that in our Ecuador location, A. iphiclus (model) were 84 times more abundant than A. serpa (mimic), and in Costa Rica A. iphiclus were only approximately 1.6 times more abundant, using adult data (electronic supplementary material, table S2a). Checa et al. [47] found an even more extreme difference between A. iphiclus and A. serpa abundances at this same Ecuador field location in 2002–2003 (using similar trapping techniques), where 322 A. iphiclus were reported and only one A. serpa over the course of 13 months. Although our abundance records show mimic A. serpa as present in both Ecuador and Costa Rica, it appears A. serpa is rarely attracted to bait traps at either field site. Therefore, although we acknowledge the potential bias in Ecuador by relying on trapping data in the year prior to our predation study, abundance estimates from hand-collecting efforts during July–October 2015 at this same field site are consistent with the trapping data. Overall, our data show a strong correlation between butterfly abundances in the field and avian attacks on models. Below we consider our results in light of prior work on frequency-dependent selection and imperfect mimicry, with the caveat that replicating this study in other locations would be useful, and that other sources of variation, such as predator community differences, seasonal variation or ecological correlates may be relevant.
(a). Frequency-dependent selection and the evolution of imperfect Batesian mimicry
Many theoretical models of Batesian mimicry make the intuitive prediction that predator attack rate on palatable mimics should increase as the ratio of mimics-to-models increases (models summarized in [4]). Generally, Batesian mimicry is expected to be ineffective when palatable mimics are more common than unpalatable models. However, our results suggest that a relative frequency of 38% (palatable mimics : models in Costa Rica) is sufficient to allow avian predators to learn to discriminate between the two species, thus causing a breakdown in Batesian mimicry. This is contrary to work done by Brower [9] that showed a much higher frequency of mimics (60%) still allowed mimic protection from predators. Mimetic species tend to co-occur with their models [1], and previous studies have provided strong evidence that the absence of an unpalatable model [11] dissolves most protection benefits for a palatable mimic, except in instances when mimics are exposed to migratory predators that have previously encountered the model [48–50]. Our results suggest that even with a relatively high abundance of the unpalatable model, any protection extended to mimics is, at best, weak.
The consequences of the putative mimic (A. serpa) not having wing patterns that perfectly resemble the model add to the complexity of frequency-dependent selection in this system. We conclude that A. serpa is an imperfect mimic of A. iphiclus given the quantitative spectral differences in the white wing patches (electronic supplementary material, table S1) [19]. Imperfect Batesian mimicry is widespread [51], but imperfect mimicry is often successful because mimetic prey may be under no further selection to improve phenotypic similarity to the model [52,53]. This may be the case in Ecuador, where the spectral differences (in the form of JNDs) between wing white patches in A. serpa (mimic) and A. iphiclus (model) are higher than in Costa Rica (electronic supplementary material, table S1). Prior work on damselflies and snake-mimicry complexes supports this idea, and suggests that phenotypic similarity to the model is frequency-dependent, with mimics more closely resembling the model when the mimic-to-model ratio is high [12–14].
Brower [9] suggested that imperfect mimicry may arise as a consequence of a ‘breakdown’ in mimicry, as we described above for Adelpha based on model : mimic ratios. Alternatively, imperfect mimicry might reflect (i) artefacts of human perception not shared by natural signal receivers, (ii) genetic or developmental constraints, which (temporarily) limit a response to selection, (iii) relaxed selection, where imperfect mimics are as fit as perfect mimics (as suggested by our results), or (iv) an (at least locally) adaptive peak [54]. In addition, other factors such as sexual selection or kin selection may contribute to imperfect signals [5,55,56], and imperfect mimicry could be maintained in certain geographical areas due to gene flow [57]. Imperfect, but successful, Batesian mimicry, as we observe in Ecuador, might also be correlated with mimic profitability [58], model abundance and unprofitability [59,60], or the availability of alternative prey (discussed below, [61]). Many studies, however, have found results consistent with predictions of relaxed selection, which seems the most likely explanation for imperfect mimicry in Ecuadorian Adelpha.
(b). Influence of prey availability and seasonality on predator behaviour
In addition to frequency-dependent dynamics and relative mimetic perfection, other important factors may contribute to a predator's decision to attack mimetic prey in a given geographical location. For example, the presence of alternative palatable prey can affect predator decision-making in both Batesian and Müllerian mimicry systems [7,61–68], and arthropod abundance and richness can vary depending on the season in the tropics [69]. Predators living in the presence of a superabundance of food may avoid relatively distasteful prey altogether [70], while they may choose to forage on weakly distasteful prey in times of energetic need [4,71]. Lindström et al. [61] showed that when alternative prey were scarce, imperfect Batesian mimics were selected out of the population, whereas abundantly available alternative prey relaxed selection against imperfect mimics. In Ecuador, where mimicry between A. iphiclus and A. serpa is effective, the overall butterfly predation rate was quite low compared to Costa Rica. This suggests that relaxed selection against imperfect mimics could potentially reflect the abundance of alternative edible prey options (e.g. orthopterans, spiders and other arthropods including butterflies) available to insectivorous predators. However, the differences in our predation rates could also be due to variation in bird communities themselves, which may be at lower densities in the Amazon [72].
Seasonality (co-occurrence) of model versus mimic can also be a significant component affecting a predator's decision to attack certain prey, which is connected to predator learning and exposure (see also [73]). Our abundance data in Costa Rica indicate seasonality of both A. serpa and A. iphiclus. Thus, seasonal variation in the relative abundance of A. serpa (mimic) might favour efforts by predators to distinguish between the two convergent species. On the other hand, many avian predators have long lifespans that exceed the average adult butterfly lifetime, and observations suggest vertebrate predators may remember unpleasant experiences with unpalatable prey for over a year [74,75]. Therefore, it would be useful to repeat our study at the Costa Rica field site when A. iphiclus (model) are relatively more abundant to test whether both species are equally avoided.
(c). Palatabiltiy versus profitabilty
A final crucial element to consider with respect to the evolution of imperfect Batesian mimics under variable model : mimic frequencies is how model capture cost varies in relation to its distastefulness [4,59] (but see also [76]). Theory predicts that predators are more likely to avoid prey that resemble an unprofitable model species when the cost of pursuing that species is very high. It is possible Adelpha might be relatively palatable, but unprofitable to pursue given capture costs. Even though the degree of adult capture cost for Adelpha is not well documented, it has previously been suggested that Adelpha might gain anti-predator protection via their rapid escape ability and unpredictable flight patterns [21,55,77]. In this case, it is not clear whether A. serpa (mimic) is less difficult to capture than A. iphiclus (model) (S. Finkbeiner 2016, personal observations), and literature [21] suggests that there are certain behavioural similarities between A. serpa and A. iphiclus. For example, males of both species share similar flight patterns and territorial perching tendencies [21] (S. Finkbeiner 2016, personal observations).
It seems far more likely that palatability varies between these two species, which might influence capture costs. Host plant breadth is extensive among Adelpha, and it has previously been hypothesized that Rubiaceae-feeding species are unpalatable, based on the alkaloids present in many species of Rubiaceae [23–25], and serve as models for species feeding on other plant families. In our Costa Rica field location, larval host plant records for both A. iphiclus and A. serpa follow this pattern where A. iphiclus (model) immatures have been found feeding on Rubiaceae plants, and A. serpa (mimic) on Melastomataceae and Malvaceae (C. Rush and R. Hill 2016, personal observations). Although there is indirect evidence that A. iphiclus may indeed possess toxins initially obtained from host plants [20], laboratory experiments analysing and identifying these toxins and their concentrations in adults have yet to be undertaken, and it is unclear whether adult toxicity and host preference varies from location to location (e.g. [78]). Therefore, additional information about the specific toxicity and capture costs of Adelpha could help clarify mimicry dynamics in this system.
5. Conclusion
A multitude of factors contribute to an avian predator's decision to avoid, or attack, a butterfly that phenotypically resembles an unprofitable species. Successful Batesian mimicry, even if imperfect, is dependent upon the relative frequencies of unpalatable models and palatable mimics. Our results using two different geographical locations demonstrate that when the model is significantly more abundant than the mimic, imperfect Batesian mimics are protected by resembling the model phenotype, whereas this protection breaks down as mimics become more common. Our results illustrate how imperfect or imprecise mimicry in populations with variable model : mimic ratios affects selection on Batesian mimics, adding to our understanding of the complex dynamics fuelling selection on mimetic phenotypes in animals. In Adelpha, where convergence is widespread, such frequency-dependent dynamics may drive divergence among geographically distinct populations, and therefore may contribute to the rapid diversification observed in this group [20,22].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Evan Kristiansen, Audrey Tjahjadi and Gulchin Isayeva for aid in butterfly model construction; Nathalia Artieda, Daniela Collaguazo, Dennise Galarza and Karina Torres for field assistance with predation experiments; Ronald Vargas, Dean Leonard, Deborah Walker, Gisela Zamora, Danilo Villegas, Gabriel Uertas and Flor Castantes for aid in butterfly collecting and trapping; Chris Schneider for providing us the fibre-optic spectrometer; Carlos de la Rosa and the Organization for Tropical Studies (OTS) and the Pontifica Universidad Católica (PUCE) Yasuní Research Station, for facilitating use of field sites and research facilities; El Ministerio del Ambiente, Energía, y Telecomunicaciones (MINAET, Costa Rica) for Costa Rica research permit approval; and the Ministerio del Ambiente, the Instituto Nacional de Biodiversidad and Santiago Villamarín for assistance in obtaining permits for field research in Ecuador.
Data accessibility
Data have been deposited with Dryad Digital Repository [79].
Authors' contributions
S.D.F. and S.P.M. conceived of the study. S.D.F. designed butterfly models, carried out field predation experiments, performed statistical analyses, wrote the manuscript and created the figures. P.A.S. and S.N. provided Ecuador abundance data and aided in field experiments. C.E.R. and R.I.H. provided Costa Rica abundance data and butterfly samples. A.D.B. calculated butterfly model discriminabilities and designed butterfly models. A.D.B., C.E.R., M.R.K., R.I.H., K.R.W., P.A.S. and S.P.M. contributed project advice and edited the manuscript.
Competing interests
We have no competing interests.
Funding
This study was funded by the National Science Foundation (NSF) Dimensions of Biodiversity grant no. DEB-1342712 to S.P.M., DEB-1342759 to A.D.B., DEB-1342706 to R.I.H., DEB-1342705 to K.R.W. and DEB-1342790 to M.R.K.
References
- 1.Bates HW. 1862. Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 23, 495–566. ( 10.1111/j.1095-8312.1981.tb01842.x) [DOI] [Google Scholar]
- 2.Cott HB. 1940. Adaptive coloration in animals. London, UK: Methuen. [Google Scholar]
- 3.Edmunds M. 1974. Defence in animals: a survey of anti-predator defences. Harlow, UK: Longman. [Google Scholar]
- 4.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Turner JRG. 1978. Why male butterflies are non-mimetic: natural selection, sexual selection, group selection, modification and sieving. Biol. J. Linn. Soc. 10, 163–206. ( 10.1111/j.1095-8312.1978.tb00023.x) [DOI] [Google Scholar]
- 6.Owen RE, Owen ARG. 1984. Mathematical paradigms for mimicry: recurrent sampling. J. Theoretic. Biol. 109, 217–247. ( 10.1016/S0022-5193(84)80004-1) [DOI] [Google Scholar]
- 7.Getty T. 1985. Discriminability and the sigmoid functional response: how optimal foragers could stabilize model–mimic complexes. Am. Nat. 125, 239–256. ( 10.1086/284339) [DOI] [Google Scholar]
- 8.Speed MP, Turner JRG. 1999. Learning and memory in mimicry. II. Do we understand the mimicry spectrum? Biol. J. Linn. Soc. 67, 281–312. ( 10.1111/j.1095-8312.1999.tb01935.x) [DOI] [Google Scholar]
- 9.Brower JV. 1960. Experimental studies of mimicry. IV. The reactions of starlings to different proportions of models and mimics. Am. Nat. 94, 271–282. ( 10.1086/282137) [DOI] [Google Scholar]
- 10.Huheey JE. 1980. Studies in warning coloration and mimicry. VIII. Further evidence for a frequency-dependent model of predation. J. Herpetology. 14, 223–230. ( 10.2307/1563543) [DOI] [Google Scholar]
- 11.Pfennig DW, Harcombe WR, Pfennig KS. 2001. Frequency-dependent Batesian mimicry: predators avoid look-alikes of venomous snakes only when the real thing is around. Nature 410, 323 ( 10.1038/35066628) [DOI] [PubMed] [Google Scholar]
- 12.Harper GR Jr, Pfennig DW. 2007. Mimicry on the edge: why do mimics vary in resemblance to their model in different parts of their geographical range? Proc. R. Soc. B 274, 1955–1961. ( 10.1098/rspb.2007.0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi DW, Pfennig DW. 2010. High-model abundance may permit the gradual evolution of Batesian mimicry: an experimental test. Proc. R. Soc. B 277, 1041–1048. ( 10.1098/rspb.2009.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iserbyt A, Bots J, Van Dongen S, Ting JJ, Van Gossum H, Sherratt TN. 2011. Frequency-dependent variation in mimetic fidelity in an intraspecific mimicry system. Proc. R. Soc. B 278, 3116–3122. ( 10.1098/rspb.2011.0126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brower JV. 1958. Experimental studies of mimicry in some North American butterflies. 1. The monarch, Danaus plexippus, and Viceroy, Limenitis achippus archippus. Evolution 12, 32–47. ( 10.2307/2405902) [DOI] [Google Scholar]
- 16.Brower JV. 1958. Experimental studies of mimicry in some North American butterflies. 2. Battus philenor and Papilio troilus, P. polyxenes, and P. glaucus. Evolution 12, 123–136. ( 10.2307/2406023) [DOI] [Google Scholar]
- 17.Brower JV. 1958. Experimental studies of mimicry in some North American butterflies. 3. Danaus gilippus berenice and Limenitis archippus floridensis. Evolution 12, 273–285. ( 10.2307/2405851) [DOI] [Google Scholar]
- 18.Platt AP, Coppinger RP, Brower LP. 1971. Demonstration of selective advantage of mimetic Limenitis butterflies presented to caged avian predators. Evolution 25, 692–701. ( 10.2307/2406950) [DOI] [PubMed] [Google Scholar]
- 19.Finkbeiner SD, Briscoe AD, Mullen SP. 2017. Complex dynamics underlie the evolution of imperfect wing pattern convergence in butterflies. Evolution 71, 949–959. ( 10.1111/evo.13165) [DOI] [PubMed] [Google Scholar]
- 20.Ebel ER, DaCosta JM, Sorenson MD, Hill RI, Briscoe AD, Willmott KR, Mullen SP. 2015. Rapid diversification associated with ecological specialization in Neotropical Adelpha butterflies. Mol. Ecol. 24, 2392–2405. ( 10.1111/mec.13168) [DOI] [PubMed] [Google Scholar]
- 21.Willmott KR. 2003. The genus Adelpha: its systematics, biology, and biogeography (Lepidoptera: Nymphalidae: Limenitidini). Gainseville, FL: Scientific Publishers. [Google Scholar]
- 22.Mullen SP, Savage WK, Wahlberg N, Willmott KR. 2011. Rapid diversification and not clade age explains high diversity in neotropical Adelpha butterflies. Proc. R. Soc. B 278, 1777–1785. ( 10.1098/rspb.2010.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmeller T, Wink M. 1998. Utilization of alkaloids in modern medicine. In Alkaloids: biochemistry, ecology, and medicinal applications (eds Roberts MF, Wink M), pp. 435–459. New York, NY: Plenum Press. [Google Scholar]
- 24.Soto-Sobenis A, Castillo B, Delgado A, Gonzales A, Montenegro R. 2001. Alkaloid screening of herbarium samples of Rubiaceae from Panama. Pharm. Biol. 39, 161–169. ( 10.1076/phbi.39.3.161.5925) [DOI] [Google Scholar]
- 25.Kessler A, Baldwin IT. 2002. Plant responses to insect herbivory: the emerging molecular analysis. Ann. Rev. Plant Biol. 53, 299–328. ( 10.1146/annurev.arplant.53.100301.135207) [DOI] [PubMed] [Google Scholar]
- 26.Aiello A. 1984. Adelpha (Nymphalidae): deception on the wing. Psyche 91, 1–45. ( 10.1155/1984/87930) [DOI] [Google Scholar]
- 27.Poulton EB. 1884. Notes upon, or suggested by, the colours, markings, and protective attitudes of certain lepidopterous larvae and pupae, and of a phytophagous hymenopterous larva. Trans. Ent. Soc. Lond. 32, 27–60. ( 10.1111/j.1365-2311.1884.tb01597.x) [DOI] [Google Scholar]
- 28.Allen JA, Clarke BC. 1984. Frequency dependent selection: homage to E. B. Poulton. Biol. J. Linn. Soc. 23, 15–18. ( 10.1111/j.1095-8312.1984.tb00802.x) [DOI] [Google Scholar]
- 29.Stobbe N, Schaefer HM. 2008. Enhancement of chromatic contrast increases predation risk for striped butterflies. Proc. R. Soc. B 275, 1535–1541. ( 10.1098/rspb.2008.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrill RM, Wallbank RWR, Bull V, Salazar PC, Mallet J, Stevens M, Jiggins CD. 2012. Disruptive ecological selection on a mating cue. Proc. R. Soc. B 279, 4907–4913. ( 10.1098/rspb.2012.1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seymoure BM, Aiello A. 2015. Keeping the band together: evidence for false boundary disruptive coloration in a butterfly. J. Evol. Biol. 28, 1618–1624. ( 10.1111/jeb.12681) [DOI] [PubMed] [Google Scholar]
- 32.Dell'Aglio DD, Stevens M, Jiggins CD. 2016. Avoidance of an aposematically coloured butterfly by wild birds in a tropical forest. Ecol. Entomol. 41, 627–632. ( 10.1111/een.12335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkbeiner SD, Briscoe AD, Reed RD. 2012. The benefit of being a social butterfly: communal roosting deters predation. Proc. R. Soc. B 279, 2769–2776. ( 10.1098/rspb.2012.0203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkbeiner SD, Briscoe AD, Reed RD. 2014. Warning signals are seductive: relative contributions of color and pattern to predator avoidance and mate attraction in Heliconius butterflies. Evolution 68, 3410–3420. ( 10.1111/evo.12524) [DOI] [PubMed] [Google Scholar]
- 35.Finkbeiner SD, Fishman DA, Osorio D, Briscoe AD. 2017. Ultraviolet and yellow reflectance but not fluorescence is important for visual discrimination of conspecifics by Heliconius erato. J. Exp. Biol. 220, 1267–1276. ( 10.1242/jeb.153593) [DOI] [PubMed] [Google Scholar]
- 36.Kelber A, Vorobyev M, Osorio D. 2003. Animal colour vision: behavioral tests and physiological concepts. Biol. Rev. 78, 81–118. ( 10.1017/S1464793102005985) [DOI] [PubMed] [Google Scholar]
- 37.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B. 265, 351–358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsson P, Lind O, Kelber A. 2015. Bird colour vision: behavioral thresholds reveal receptor noise. J. Exp. Biol. 218, 184–193. ( 10.1242/jeb.11187) [DOI] [PubMed] [Google Scholar]
- 39.Hart NS, Partridge JC, Cuthill IC, Bennett ATD. 2000. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Physiol. A. 186, 375–387. ( 10.1007/s003590050437) [DOI] [PubMed] [Google Scholar]
- 40.Endler JA. 1993. The color of light in forests and its implications. Ecol. Monographs 63, 1–27. ( 10.2307/2937121) [DOI] [Google Scholar]
- 41.Beccaloni GW. 1997. Ecology, natural history and behaviour of Ithomiine butterflies and their mimics in Ecuador (Lepidoptera: Nymphalidae: Ithomiine). Trop. Lepid. 8, 103–124. [Google Scholar]
- 42.Outomuro D, Ángel-Giraldo P, Corral-Lopez A, Realpe E. 2016. Multitrait aposematic signal in Batesian mimicry. Evolution 70, 1596–1608. ( 10.1111/evo.12963) [DOI] [PubMed] [Google Scholar]
- 43.Mallet J, Barton NH. 1989. Strong natural selection in a warning-color hybrid zone. Evolution 43, 421–431. ( 10.2307/2409217) [DOI] [PubMed] [Google Scholar]
- 44.Kapan DD. 2001. Three-butterfly system provides a field test of Müllerian mimicry. Nature 409, 338–340. ( 10.1038/35053066) [DOI] [PubMed] [Google Scholar]
- 45.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 46.Fox J, Weisberg S. 2011. Cox proportional-hazards regression for survival data in R. In An R companion to applied regression (eds J Fox, S Weisberg), pp. 1–20. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- 47.Checa MF, Barragán A, Rodríguez J, Christman M. 2009. Temporal abundance patterns of butterfly communities (Lepidoptera: Nymphalidae) in the Ecuadorian Amazonia and their relationship with climate. Ann. Soc. Entomol. Fr. 45, 470–486. ( 10.1080/00379271.2009.10697630) [DOI] [Google Scholar]
- 48.Poulton EB. 1909. The value of colour in the struggle for life. In Darwin and modern science; essays in commemoration of the centenary of the birth of Charles Darwin and of the fiftieth anniversary of the publication of the Origin of Species (ed. Seward AC.), pp. 207–227. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 49.Carpenter GDH, Ford EB. 1933. Mimicry. London, UK: Methuen and Co. [Google Scholar]
- 50.Waldbauer GP. 1988. Aposematism and Batesian mimicry: measuring mimetic advantage in natural habitats. Evol. Biol. 22, 227–259. ( 10.1007/978-1-4613-0931-4_5) [DOI] [Google Scholar]
- 51.Edmunds M. 2000. Why are there good and poor mimics? Biol. J. Linn. Soc. 70, 459–466. ( 10.1111/j.1095-8312.2000.tb01234.x) [DOI] [Google Scholar]
- 52.Schmidt RS. 1958. Behavioural evidence on the evolution of Batesian mimicry. Anim. Behav. 6, 129–138. ( 10.1016/0003-3472(58)90042-3) [DOI] [Google Scholar]
- 53.Duncan CJ, Sheppard PM. 1965. Sensory discrimination and its role in the evolution of Batesian mimicry. Behavior. 24, 269–282. ( 10.1163/156853965X00066) [DOI] [PubMed] [Google Scholar]
- 54.Kikuchi DW, Pfennig DW. 2013. Imperfect mimicry and the limits of natural selection. Q. Rev. Biol. 88, 297–315. ( 10.1086/673758) [DOI] [PubMed] [Google Scholar]
- 55.Mallet J, Singer MC. 1987. Individual selection, kin selection, and the shifting balance in the evolution of warning colours: the evidence from butterflies. Biol. J. Linn. Soc. 32, 337–350. ( 10.1111/j.1095-8312.1987.tb00435.x) [DOI] [Google Scholar]
- 56.Su S, Lim M, Kunte K. 2015. Prey from the eyes of predators: color discriminability of aposematic and mimetic butterflies from an avian visual perspective. Evolution 69, 2985–2994. ( 10.1111/evo.12800) [DOI] [PubMed] [Google Scholar]
- 57.Harper GR, Pfennig DW. 2008. Selection overrides gene flow to break down maladaptive mimicry. Nature 451, 1103–1106. ( 10.1038/nature06532) [DOI] [PubMed] [Google Scholar]
- 58.Penney HD, Hassall C, Skevington JH, Abott KR, Sherratt TN. 2012. A comparative analysis of the evolution of imperfect mimicry. Nature 483, 461–464. ( 10.1038/nature10961) [DOI] [PubMed] [Google Scholar]
- 59.Lindström L, Alatalo RV, Mappes J. 1997. Imperfect Batesian mimicry: the effects of the frequency and the distastefulness of the model. Proc. R. Soc. Lond. B 264, 149–153. ( 10.1098/rspb.1997.0022) [DOI] [Google Scholar]
- 60.Sherratt TN, Peet-Paré CA. 2017. The perfection of mimicry: an information approach. Phil. Trans. R. Soc. B 372, 20160340 ( 10.1098/rstb.2016.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindström L, Alatalo RV, Lyytinen A, Mappes J. 2004. The effect of alternative prey on the dynamics of imperfect Batesian and Müllerian mimicries. Evolution 58, 1294–1302. ( 10.1111/j.0014-3820.2004.tb01708.x) [DOI] [PubMed] [Google Scholar]
- 62.Holling CS. 1965. The functional response of predators to prey density, and its role in mimicry and population regulation. Mem. Ent. Soc. Canada. 45, 1–60. ( 10.4039/entm9745fv) [DOI] [Google Scholar]
- 63.Emlen JM. 1968. Batesian mimicry: a preliminary theoretical investigation of quantitative aspects. Am. Nat. 102, 235–241. ( 10.1086/282540) [DOI] [Google Scholar]
- 64.Dill LM. 1975. Calculated risk-taking by predators as a factor in Batesian mimicry. Can. J. Zool. 53, 1614–1621. ( 10.1139/z75-195) [DOI] [Google Scholar]
- 65.Luedeman JK, McMorris FR, Warner DD. 1981. Predators encountering a model–mimic system with alternative prey. Am. Nat. 117, 1040–1048. ( 10.1086/283794) [DOI] [Google Scholar]
- 66.Kokko H, Mappes J, Lindström L. 2003. Alternative prey can change model–mimic dynamics between parasitism and mutualism. Ecol. Lett. 6, 1068–1076. ( 10.1046/j.1461-0248.2003.00532.x) [DOI] [Google Scholar]
- 67.Sherratt TN. 2003. State-dependent risk-taking in systems with defended prey. Oikos 103, 93–100. ( 10.1034/j.1600-0706.2003.12576.x) [DOI] [Google Scholar]
- 68.Ihalainen E, Rowland HM, Speed MP, Ruxton GD, Mappes J. 2012. Prey community structure affects how predators select for Müllerian mimicry. Proc. R. Soc. B 279, 2099–2105. ( 10.1098/rspb.2011.2360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basset Y, et al. 2015. Arthropod distribution in a tropical rainforest: tackling a four dimensional puzzle. PLoS ONE 10, e0144110 ( 10.1371/journal.pone.0144110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Ruiter L. 1959. Some remarks on problems of the ecology and evolution of mimicry. Archives Neerlandaises de Zoologie. 13, 351–368. [Google Scholar]
- 71.Sheppard PM. 1975. Natural selection and heredity. London, UK: Longman. [Google Scholar]
- 72.Thiollay J-M. 1994. Structure, density and rarity in an Amazonian rainforest bird community. J. Trop. Ecology 10, 449–481. ( 10.1017/S0266467400008154) [DOI] [Google Scholar]
- 73.Srygley RB, Kingsolver JG. 1998. Red-wing blackbird reproductive behaviour and the palatability, flight performance, and morphology of temperate pierid butterflies (Colias, Pieris, and Pontia). Biol. J. Linn. Soc. 64, 41–55. ( 10.1111/j.1095-8312.1998.tb01532.x) [DOI] [Google Scholar]
- 74.Mostler G. 1935. Beobachtungen zur frage der wespen-mimikry. Zeitschrift für Morphologie und Ökologie der Diere. 29, 381–454. ( 10.1007/BF00403719) [DOI] [Google Scholar]
- 75.Rothschild M. 1964. An extension of Dr. Lincoln Brower's theory on bird predation and food specificity, together with some observations on bird memory in relation to aposematic colour patterns. Entomologist. 97, 73–78. [Google Scholar]
- 76.Rowland HM, Ihalainen E, Lindström L, Mappes J, Speed MP. 2007. Co-mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67. ( 10.1038/nature05899) [DOI] [PubMed] [Google Scholar]
- 77.Pinheiro CEG. 1996. Palatability and escaping ability in Neotropical butterflies: tests with wild kingbirds (Tyrannus melancholicus, Tyrannidae). Biol. J. Linn. Soc. 59, 351–365. ( 10.1111/j.1095-8312.1996.tb01471.x) [DOI] [Google Scholar]
- 78.Ritland DB. 1995. Comparative unpalatability of mimetic viceroy butterflies (Limenitis archippus) from four south-eastern United States populations. Oecologia 103, 327–336. ( 10.1007/BF00328621) [DOI] [PubMed] [Google Scholar]
- 79.Finkbeiner SD, et al. 2018. Data from: Frequency dependence shapes the adaptive landscape of imperfect Batesian mimicry Dryad Digital Repository. ( 10.5061/dryad.cp3f820) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Finkbeiner SD, et al. 2018. Data from: Frequency dependence shapes the adaptive landscape of imperfect Batesian mimicry Dryad Digital Repository. ( 10.5061/dryad.cp3f820) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data have been deposited with Dryad Digital Repository [79].



