Abstract
Social animals often form long-lasting relationships with fellow group members, usually with close kin. In primates, strong social bonds have been associated with increased longevity, offspring survival and reproductive success. However, little is known about the fitness effects of social bonds between non-kin, especially outside of mammals. In this study, we use long-term field research on a cooperatively breeding bird, the greater ani (Crotophaga major), to ask whether adult females benefit by remaining in long-term associations with unrelated, co-breeding females. We find that females that have previously nested together synchronize their reproduction more rapidly than those nesting with unfamiliar partners, which leads to lower competition and higher fledging success. Importantly, although previous experience with a co-breeding female influenced reproductive synchrony, the degree of reproductive synchrony did not influence whether co-breeding females remained together in subsequent years, ruling out the alternate hypothesis that highly synchronized females are simply more likely to remain together. These results indicate that switching groups is costly to females, and that social familiarity improves reproductive coordination. Stable social relationships therefore have significant fitness consequences for cooperatively nesting female birds, suggesting that direct benefits alone may favour the evolution of associations between non-relatives and contribute to long-term group stability.
Keywords: cooperation, cooperative breeding, Crotophaga major, group stability, social affiliation, social bond
1. Introduction
In social animals ranging from guppies to humans, relationships between group members have profound fitness consequences [1,2]. Studies of group-living mammals, including primates [3], dolphins [4,5], bats [6] and equids [7], have found that strong and stable social bonds are correlated with increased longevity, offspring survival and immune function (reviewed in [8]). The disruption of social bonds can also have negative consequences at the group level, because changes in group membership may disrupt cooperative activities such as alloparental care [9,10], group hunting [11,12], communal territory defense [13] and reciprocal food sharing [14,15]. This is analogous to the ‘newcomer effect' documented in human social groups, in which turnover in membership can hinder group functioning and performance [16,17].
In non-human animals, however, efforts to understand the evolutionary pressures shaping group stability are often confounded by genetic relatedness between group members. Since most social groups form as extended families (and individuals often prefer to associate with close relatives) social bonds may be favoured by kin selection rather than by the direct fitness benefits of association (e.g. refs [18–23]). In primates and ungulates, disentangling the influence of kinship and social affiliations is additionally confounded by dominance rank, because high-ranking individuals often live with more close kin and have stronger social relationships than do lower-ranking individuals [24,25]. As a result, surprisingly few studies have demonstrated that stable social relationships between non-kin are adaptive [7,26].
In this study, we investigate the long-term fitness consequences of social bonds between unrelated females in the greater ani (Crotophaga major), a cooperatively breeding tropical bird. Anis live in small groups that typically consist of two or three pairs that build a shared nest in which all of the group's females lay their eggs [27]. Pairs are largely genetically monogamous as well as socially monogamous, and all of the adults in the breeding group typically contribute offspring to the mixed clutch [28]. Adults cannot recognize their own eggs or nestlings, and all group members participate in incubation, food delivery and nest defense [29–30]. Although group members are not genetic relatives [31], ani breeding groups can be extremely stable over time, lasting over a decade in the wild. Adult anis are long-lived (mark–resighting analyses suggest that adult lifespan averages between 15 and 20 years; C.R. and M.J.S. 2010–2018, unpublished data), and behavioural evidence suggests strong social bonds between same and opposite-sex group members as well as between mates [32]. On average, social groups remain together for approximately 3.5 years (range 1–11 years), indicating that most females change groups at least once in their lifetimes, and probably 2–3 times. Groups remain on the breeding territory year-round, continuing to roost, forage and display together throughout the non-breeding season [27].
Previous research on our study population in Panama has found that group nesting is favoured by the direct fitness benefits of cooperative nest defense. Larger groups are more likely to acquire and defend safe, high-quality nesting territories that are inaccessible to terrestrial nest predators such as snakes and monkeys, and they are better able to directly defend eggs and nestlings against predation attempts [31,33]. Single pairs rarely attempt to nest alone, and have never been observed to successfully raise young [31]. However, group nesting also has significant fitness costs. At the beginning of the laying period, the first egg to be laid in the communal nest is always removed by another female in the group—a female who, by definition, has not yet started laying. Each female stops removing eggs from the nest once she has laid her own first egg, presumably to avoid destroying her own, so eggs can only accumulate in the communal clutch once all of the groups' females have started laying (figure 1). This rule of thumb—remove eggs before laying, then accept eggs after laying—leads to predictable patterns of egg loss. The first female to start laying always loses at least one egg, and sometimes several; whereas the last-laying female never loses any [31,34]. The number of eggs lost by early-laying females therefore depends on two factors: the number of breeding females in the group, and their degree of reproductive synchrony [34,35].
Figure 1.
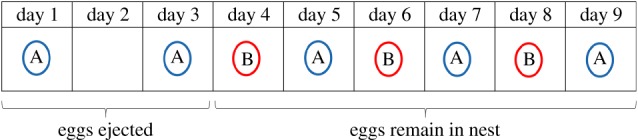
Schematic representation of egg ejection and reproductive synchrony in a typical ani breeding group with two females. Each female lays one egg every two days. The laying period begins on day 1 when the first female (A) lays her first egg in the shared nest. The other female (B) ejects female A's eggs until she herself has laid her own first egg (in this case, on day 4). Once both females have laid at least one egg, ejection ceases and eggs accumulate in the nest. In this case, 3 days elapsed between female A's first egg and female B's first egg, and female A lost 2 eggs to ejection. (Online version in colour.)
In this paper, we use 11 years of data from our long-term study population of greater anis in Panama to investigate correlations between social group stability, reproductive synchrony and individual fitness. Uniquely, this longitudinal dataset allows us to explore the effects of social affiliations while controlling for the potential confounds of age, experience and territory quality. Although groups contain both males and females (and both sexes may remain in the same group for many years [32]), we focus our analyses on females. Not only are the competitive costs of communal nesting borne largely by females [36,37], but we also possess more data on individual female reproductive histories, allowing stronger inferences regarding the selective pressures shaping female fitness. We test the hypotheses that females that have previously nested together will exhibit higher reproductive synchrony and lower competition than females nesting together for the first time. We predict that females nesting with familiar partners should have higher reproductive success (lose fewer eggs to intra-group competition and produce more offspring) than those nesting with unfamiliar partners. However, this correlation could also emerge if more highly synchronized females are simply more likely to remain in the same group over time, such that synchrony is the cause of stability rather than the effect. To eliminate this possibility, therefore, we also test whether reproductive synchrony in one year influences the likelihood that female group members remain together in the subsequent year.
2. Material and methods
(a) Data collection
We collected data on group membership and reproductive output from a genotyped study population of greater anis in the Barro Colorado Nature Monument, Panama (9.154542, −79.845540). Greater anis are large (150–200 g) tropical cuckoos that inhabit forested waterways from central Panama to northern South America; they are obligately communal and build nests in emergent vegetation along lake and river edges, or in tree branches overhanging the water. Between 40 and 60 communal ani nests were located and monitored each year between 2007 and 2017, representing greater than 90% of nesting attempts within the study area [28]. Monitoring protocols for this long-term project are available in [27–32]. Briefly, nests were checked daily prior to laying and during the laying period, every 2–3 days during the 12-day incubation period, and daily during the first 6 days of the nestling period to determine daily predation rates and individual reproductive output. Eggs were individually numbered in the order in which they were laid, genetically sampled for egg maternity (see below), and recorded as ejected by other group members, depredated, infertile or hatched [27]. Initiation date was considered to be the ordinal date on which the first egg was laid in the communal nest, and the time required to synchronize reproduction was considered to be the number of days elapsed between the onset of laying for the first and last female in the nesting group. Laying period was recorded as the number of days from the laying of the first egg until the onset of incubation; this varied across groups as a function of synchronization time and clutch size. Incubation period was 12 days for all nests [27], and nestling period was considered to be 6 days, the age at which nestlings are capable of leaving the nest. Previous studies have shown that the location of the nest site is an important determinant of nest success (nests in shoreline vegetation are at an increased risk of depredation compared with nests in emergent vegetation that is completely surrounded by water [31]), so nest-site quality was recorded as a binary variable (0 = shoreline, 1 = emergent) and included as a potential predictor variable in initial analyses.
(b). Genetic analyses
Group member identity was assigned by using both observational data of colour-banded females (primarily from 2007–2011) and genetic identification of egg maternity (primarily from 2009–2017). In cases where adult females were not colour-banded, we used maternal genotypes from eggs to determine whether a female was part of the same social group in consecutive years. Genomic DNA was extracted from blood samples of adults and nestlings with Qiagen DNEasy Blood and Tissue Kits (2006–2010 samples) or Omega Bio-Tek EZNA Tissue Kits (2011–2015 samples) using the manufacturers' protocols. From 2007–2011, 25–50 breeding adults per year were captured in mist nets, individually colour-banded, and genetically sampled by brachial venipuncture (n = 225). Maternal genomic DNA was non-destructively sampled from freshly laid eggs and destructively sampled from the membranes of ejected eggs [38,39]. DNA was extracted from eggshell membranes with Omega Bio-Tek EZNA. Forensic DNA kits using the manufacturer's protocols, and maternal identity of eggs and nestlings was cross-validated using techniques described in [39]. Each individual was genotyped using a set of 12 highly polymorphic microsatellite markers developed for this species [40]. The measured typing error rate was 0.7%.
(c). Statistical analyses
We constructed models to identify factors predicting: (1) the degree of reproductive synchrony between co-breeding females in a group, measured as the number of days required for all females in a group to begin laying; (2) the number of eggs lost by early-laying females to ejection by other group members (a direct correlate of the time required to synchronize reproduction; (3) daily survival rates of nests (the daily probability that a nest would escape destruction by a predator); and (4) the probability that a group would lay a second clutch if the first clutch was depredated. The goal of these models was to ask whether the overall stability of the social group and/or the duration of social bonds within groups influenced the reproductive output of individual females. Group identity was included as a random effect in all models to account for repeated measures across years. For all analyses, best-fit models were selected using a ‘best-subsets' approach, in which initial models included all terms and were compared with all possible models using subsets of the terms. Models were evaluated with Akaike's information criterion corrected for finite sample size (AICc) [41]. Models within two AICc units of the top model (ΔAICc = 0) were candidates of potential explanatory value; however, models within two AICc units of the top model that differed from a higher-ranking model by the addition of one parameter were rejected as uninformative, as recommended by Arnold [42]. Full model results and overall significance tests for models are presented in electronic supplementary material; inferences from models were made only when the overall model was significant. Analyses were conducted in STATA 14.
We first constructed separate sets of generalized linear mixed models with degree of reproductive synchrony and number of eggs lost as response variables, and 10 candidate predictors: number of females in the nesting group, standardized initiation date, age of first-laying female, age of second-laying female, age of third-laying female, age of oldest female, mean female age within the nesting group, nest-site type (emergent versus shoreline vegetation), group stability and duration of social bonds. Group stability was a binary variable (reflecting only whether co-breeding females had previously nested together), whereas duration of social bonds was a continuous variable (the number of years over which co-breeding females had nested together). Top candidate models and model selection process is shown in electronic supplementary material, tables S1–S2.
We used logistic exposure models [43] to estimate daily survival and mortality probabilities of nests for each period of the nesting cycle (laying, incubation and nestling) using group size, initiation date, territory quality and group stability as predictors. Cumulative survival probabilities for each period were then estimated by raising model-averaged daily survival probabilities to an exponent equal to the length of each period. Finally, we used mixed-effects logistic regression models to predict the binary probability that a group would lay a second clutch of eggs following depredation (using a subset of data for groups whose first clutch was depredated; n = 138 attempts). The same set of 10 candidate predictors (number of females in the nesting group, standardized initiation date, age of first-laying female, age of second-laying female, age of third-laying female, age of oldest female, mean female age within the nesting group, nest-site type (emergent versus shoreline vegetation), group stability and duration of social bonds) were used in initial models and final best-fit models were chosen using AICc.
3. Results
(a). Duration of social relationships
We monitored 233 nesting attempts by 90 communally breeding groups of greater anis over 11 years (2007–2017) in a study population in central Panama. The dataset included 209 females for whom we obtained 1–11 years of reproductive data per individual (mean ± s.d. = 3.96 ± 2.94), for a total of 765 female-years. Breeding groups were composed of either two (n = 194, 83%) or three (n = 39, 17%) socially monogamous pairs. Social bond duration (defined as the number of years over which there were no transitions in group membership, such that no females joined or left the nesting group) ranged from 1 to 11 years and followed a negative binomial distribution: the majority of nesting groups (62%) experienced a change in group membership after 1 year, but a significant minority of nesting groups remained stable for multiple years (31% of groups experienced no change in membership for 2–5 years, and 7% of groups experienced no change in membership for 6–11 years). However, our measures of the duration of social bonds are underestimates, because observations began on pre-existing groups (already together for an unknown duration), and because groups may persist for longer than the study period. Therefore, we used both social bond duration and the more accurate measure of year-to-year stability (whether a group experienced a change in group membership from year n to year n + 1) as predictor variables in subsequent analyses. Of 62 groups for which we had two or more consecutive years of data on group membership at the same nesting site (n = 207 group-years), we observed 95 instances in which a transition occurred between consecutive years, and 112 instances in which no transitions in group membership occurred between consecutive years. Dyadic relatedness between co-breeding females was not significantly different from background genetic relatedness in the population, indicating a lack of kin structure (data for groups from 2007–2009 in [31]; C.R. and M.J.S. 2010–2017, unpublished data).
(b). Group stability predicts reproductive synchrony
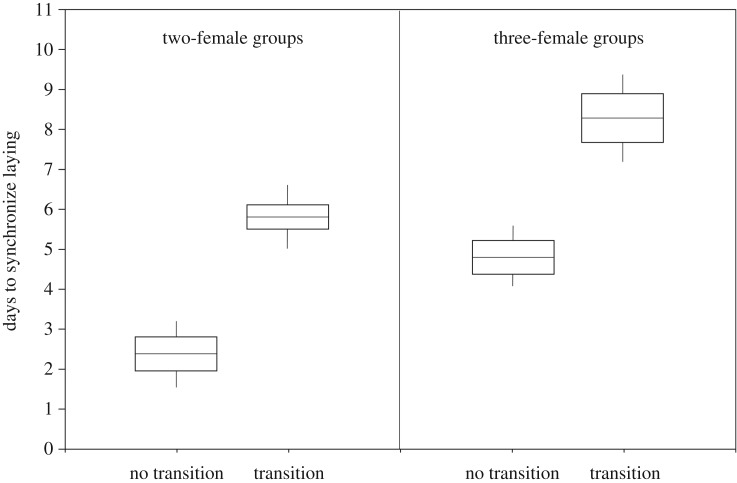
The time needed to synchronize reproduction, measured as the number of days elapsed between the onset of laying for the first and last female in the nesting group, ranged from 1 to 20 days and was longer in three-female groups than in two-female groups (three-female groups: n = 39, mean ± s.e. = 7.57 ± 0.76; two-female groups: n = 195, mean ± s.e. = 3.99 ± 0.21). Controlling for the significant effect of group size, females that had previously nested together were more highly synchronized in their reproduction than those that had never before nested together (table 1, figure 2). On average, two-female groups in which the females had nested together the previous year synchronized their laying 3.34 (±0.21) days more rapidly than those in which the females had not previously nested together. Three-female groups in which all three females had nested together the previous year synchronized their laying 4.32 (±0.8) days more rapidly than those groups that had experienced a transition in group membership (at least one female had joined or left the group since the previous year).
Table 1.
Predictors of reproductive synchrony. Results of best-fit generalized linear mixed models predicting reproductive synchrony between females in communally nesting groups of greater anis, with ejection period (number of days elapsed between onset of laying for first and last-laying females) and number of eggs ejected as response variables. Initiation date was standardized relative to the mean for all nests in a given year, and stability was coded as a binary variable (0 = all female group members were present in the same group in the prior year, 1 = at least one female joined or left the group since the prior year). Italicized values are statistically significant at p < 0.0001.
| response variable: ejection period (days) | |||||
|---|---|---|---|---|---|
| coefficient (s.e.) | Z | p | 95% CI | ||
| fixed effects | |||||
| number of females | 2.22 (0.52) | 4.29 | <0.0001 | 1.20 | 3.23 |
| age of last-laying female | 0.01 (0.08) | 0.15 | 0.88 | −0.14 | 0.16 |
| initiation date | 0.36 (0.21) | 1.72 | 0.08 | −0.05 | 0.78 |
| stability | 3.55 (0.45) | 7.93 | <0.0001 | 2.67 | 4.43 |
| intercept | −2.22 (1.20) | −1.85 | 0.07 | −4.58 | 0.14 |
| random effects | |||||
| group ID | 4.04 × 10−31 (4.21 × 10−16) | ||||
Figure 2.
Differences in the time needed to synchronize laying for communally breeding female anis, with respect to group size (two versus three females in the breeding group) and stability (stable = all females nested together the previous year; unstable = at least one female joined or left the breeding group since the previous year). The time needed to synchronize laying is given as the number of days elapsed between the onset of laying for the first and last female in the nesting group. Boxes represent model-predicted means ± s.e.m.; whiskers represent 95% confidence intervals (n = 170 two-female groups and 37 three-female groups).
Since the last-laying female in each communal group removes the eggs of early-laying females until she lays her own first egg, the number of eggs lost to intra-group competition was positively correlated with the number of days that elapsed between the onset of laying for the first and last female in the group (F1, 209 = 534.5, R2 = 0.72, p < 0.0001). Using the number of eggs lost by early-laying females as a response variable (rather than the number of days needed to synchronize reproduction) resulted in qualitatively identical results, with both group size and transitions in group membership emerging as significant predictors (table 2). In two-pair groups, a transition in group membership resulted in the first-laying female losing an additional 1.27 (±0.08) eggs to competition (relative to two-pair groups with no change in membership). In three-pair groups, a transition in group membership resulted in the first-laying female losing an additional 1.50 (±0.48) eggs to competition (relative to three-pair groups with no change in membership).
Table 2.
Predictors of reproductive synchrony. Results of best-fit generalized linear mixed models predicting reproductive synchrony between females in communally nesting groups of greater anis, with ejection period (number of days elapsed between onset of laying for first and last-laying females) and number of eggs ejected as response variables. Initiation date was standardized relative to the mean for all nests in a given year, and stability was coded as a binary variable (0 = all female group members were present in the same group in the prior year, 1 = at least one female joined or left the group since the prior year). Italicized values are statistically significant at p < 0.0001.
| response variable: number of eggs ejected | |||||
|---|---|---|---|---|---|
| coefficient (s.e.) | Z | p | 95% CI | ||
| fixed effects | |||||
| number of females | 1.65 (0.22) | 7.64 | <0.0001 | 1.23 | 2.08 |
| age of last-laying female | 0.01 (0.03) | 0.20 | 0.84 | −0.06 | 0.07 |
| initiation date | 0.03 (0.09) | 0.37 | 0.71 | −0.14 | 0.21 |
| stability | 1.30 (0.19) | 6.93 | <0.0001 | 0.93 | 1.67 |
| intercept | −2.19 (0.50) | −4.35 | <0.001 | −3.18 | −1.20 |
| random effects | |||||
| group ID | 0.05 (0.09) | ||||
For both reproductive synchrony and egg loss, group stability was a significant predictor only when it was coded as a binary variable (0 = no transition in group membership from the prior year, 1 = at least one transition in group membership from the prior year). Neither response variable was affected by the number of years that co-breeding females had nested together: The length of the partnership (measured as the number of years without a transition in group membership) was not a significant predictor in either model, and was dropped from the final best-fit models. Initiation date, female age, mean age of group members, territory quality and the difference in ages among females in the nesting group were all included in initial models, but all except the first two were dropped from final best-fit models. Initiation date (ordinal date of the first egg laid in the nest, standardized by year) and the age of the last-laying female were both retained in the final best-fit models because inclusion of these variables significantly improved model fit, but neither predictor was significant (tables 1 and 2; electronic supplementary material, tables S1–S2).
Finally, we tested whether reproductive synchrony in one year predicted the likelihood that group membership would remain stable in the subsequent year, in order to test the possibility that more highly synchronized females were a priori more likely to remain in the same social group (which could also cause a correlation between synchrony and group stability). For a subset of nests for which we had two or more consecutive years of data on group membership (n = 92), we found no significant difference in reproductive synchrony between those that subsequently experienced a transition in group membership (n = 35, mean ± s.e. = 4.85 ± 0.76 days) and those that remained stable (n = 57, 4.66 ± 0.39 days; t91 = −0.25, p = 0.81). In other words, previous experience with a co-breeding female influenced reproductive synchrony, but the degree of reproductive synchrony did not influence whether co-breeding females remained together in subsequent years.
(c). Reproductive synchrony decreases cumulative predation risk
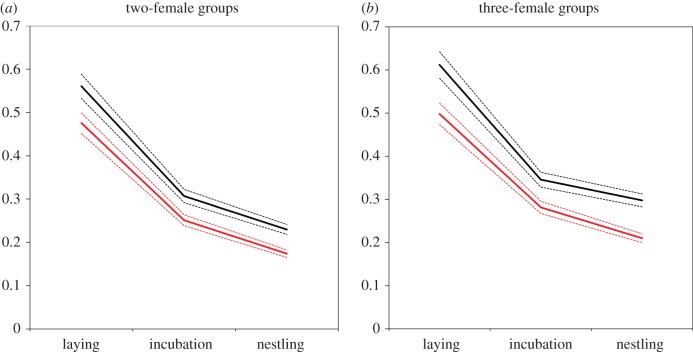
Nest predation was the most important cause of reproductive failure, accounting for approximately 97% of failed nesting attempts (n = 138 of 143 failures); primary predators included snakes (Spilotes pullatus and Pseustes poecilionotus), white-faced capuchin monkeys (Cebus capuchinus) and yellow-headed caracaras (Milvago chimachima). Controlling for group size, stable groups were more likely to fledge young than were groups that had experienced a transition in group membership (z = 2.52, p = 0.02; table 3, figure 3). However, this effect was due solely to higher reproductive synchrony in stable groups (see above), which decreased the total number of days during which the nest was exposed to predation. There were no significant differences in daily survival rate between stable and unstable groups at any stage during the nesting cycle (laying: p = 0.24; incubation: p = 0.33; nestling: p = 0.83; table 3); but the cumulative survival rate across the entire nesting cycle was higher for stable groups because the laying period was significantly shorter (table 3, figure 3).
Table 3.
Daily and cumulative nest survival probability. Model-averaged daily survival rates (DSR) and mean duration (length) in days of each period of the nesting cycle for nests of communally breeding greater anis, with respect to group size (two or three females) and stability (stable, all females nested together the previous year; unstable, at least one female joined or left the group since the previous year). Significant differences between stable and unstable groups are given in italics; asterisks indicate statistical significance at p = 0.05 (*) or p = 0.01 (**).
| laying |
incubation |
nestling |
|||||
|---|---|---|---|---|---|---|---|
| DSR | length | DSR | length | DSR | length | cumulative survival rate | |
| two-female group | |||||||
| stable | 0.957 | 8.31** | 0.945 | 12 | 0.940 | 6.31 | 0.23* |
| unstable | 0.953 | 11.64** | 0.945 | 12 | 0.941 | 6.26 | 0.17* |
| three-female group | |||||||
| stable | 0.955 | 10.33** | 0.973 | 12 | 0.975 | 6.26 | 0.29* |
| unstable | 0.961 | 14.65** | 0.972 | 12 | 0.977 | 6.25 | 0.21* |
Figure 3.
Cumulative nest survival probabilities for communally breeding groups of greater anis, with respect to group size (two-female versus three-female groups) and stage of the nesting cycle (laying, incubation, nestling). Black lines represent stable groups (all females in the group nested together in the previous year); red lines represent unstable groups (at least one female joined or left the nesting group since the previous year). Solid lines represent model-predicted cumulative survival probabilities; dashed lines represent bootstrapped 95% confidence intervals (number of bootstraps = 1000). (a) n = 170 two-female groups. (b) n = 37 three-female groups. Cumulative survival probabilities represent the likelihood of offspring (eggs or nestlings) surviving to the next stage of the nesting cycle, and are equivalent to one minus the cumulative risk of predation. (Online version in colour.)
(d). Group member age and group stability predict likelihood of re-nesting
Approximately 18% of groups laid a second clutch of eggs if their first clutch was depredated (n = 25 of 138 groups). Both age and group stability were significant predictors of the probability that the group would lay a second clutch if the first clutch of young was depredated (table 4). In this case, the duration of social bonds (the number of years that female group members had nested together) was a better predictor of re-nesting than the simple binary measure of group stability (whether or not group membership had changed because the previous year). The binary measure of group stability was not a significant predictor when the duration of social bonds was included in the model, and it was dropped from the best-fit model (electronic supplementary material, table S3). Age of individual females represented an important confounding variable for two reasons. First, ages of females were correlated within social groups, such that females tended to nest with age-matched group members (F1, 227 = 147.4, R2 = 0.39, p < 0.0001). We therefore included mean age of group members as a candidate predictor in models of re-nesting probability. Second, the mean age of group members was significantly correlated with the duration of the social group, such that groups that were stable for many years also tended to contain older members (F1, 206 = 144.4, R2 = 0.41, p < 0.0001). Both mean age and duration of social group stability were retained as candidate predictors because collinearity between these two variables was not severe (variance inflation factor = 1.67) and both were identified as significant predictors in the best-fit model, indicating that group stability affects re-nesting probability even when age is controlled for (table 4).
Table 4.
Predictors of re-nesting probability. Results of best-fit mixed-effects logistic regression models predicting the binary probability of re-nesting for communally breeding groups of greater anis, for groups whose first nesting attempt was depredated (0 = did not re-nest, 1 = re-nested). Initiation date was standardized relative to the mean for all nests in a given year, and duration of group is expressed as the number of years over which all female group members remained in the group (i.e. the number of years over which group membership was constant). Mean age is the average age (in years) of all female group members (see text for details). Italicized values are statistically significant at p < 0.05.
| coefficient (s.e.) | Z | p | 95% CI | ||
|---|---|---|---|---|---|
| fixed effects | |||||
| ejection period | −0.08 (0.16) | −0.53 | 0.60 | −0.39 | 0.23 |
| mean age | 0.37 (0.17) | 2.16 | 0.03 | 0.03 | 0.70 |
| initiation date | −1.02 (0.49) | −2.07 | 0.04 | −1.99 | −0.05 |
| duration of group | 0.52 (0.13) | 2.45 | 0.02 | 0.12 | 0.84 |
| intercept | −2.89 (1.38) | −2.09 | 0.04 | −5.61 | −0.18 |
| random effects | |||||
| group ID | 0.38 (1.79) | ||||
4. Discussion
Recent studies have provided ample evidence that stable social relationships affect the fitness of members of cooperative groups, but the vast majority of this work has been done on mammals that live in kin-based societies [18–26]. Here we find that familiarity between unrelated co-breeding female birds is a key predictor of their reproductive synchrony, significantly affecting the competitive costs associated with communal nesting as well as the cumulative predation risk for the shared nest. Importantly, our data demonstrate that social bond stability predicts reproductive synchrony, but not vice versa. We were therefore able to rule out the alternative hypothesis that co-breeding females who are more highly reproductively synchronized—due to age, physiological similarities or other reasons unrelated to social experience—are simply more likely to remain together for subsequent years, leading to group stability as a by-product of compatibility. Females who had previously nested together were also more likely to re-nest after clutch failure, increasing the probability of successful reproduction. Taken together, these data suggest that social group stability affects individual fitness in a variety of ways, primarily by influencing coordination and timing of reproduction.
Correlations between social familiarity and reproductive synchrony have also been reported in some communally breeding mammals, including bats [44], mice [45] and voles [46]. As in greater anis, the adaptive benefits of synchronized reproduction in these taxa include efficient communal brood care and reduced predation risk [44–46]. However, the proximate mechanisms by which social experience affects reproductive synchrony may differ in birds and mammals. Whereas chemical cues have been proposed to be a primary mediator of reproductive synchrony in mammals [47] (although see refs [48–49]), chemical signalling is relatively rare in birds and is largely restricted to lineages with well-developed olfactory abilities such as seabirds [50]. Instead, the neuroendocrine pathways involved in reproductive physiology appear to be stimulated primarily by visual and/or auditory cues, often in the context of behavioural displays that involve both [51,52]. In greater anis, reproductive synchrony among females may be mediated in part by stereotyped vocal displays at the communal nest, in which group members gather in a circle and call loudly for several minutes (sometimes referred to as ‘rallies' or ‘choruses' [27]). Although the functions of these displays have not been experimentally determined, the fact that displays are most frequently performed before the onset of laying (and sharply diminish in frequency after egg-laying begins [27]) is consistent with the hypothesis that they play a role in stimulating reproduction and coordinating egg-laying within the social group.
A major limitation of our dataset is that we have little information on the identity of males in social groups. Given that pairs are socially monogamous (and largely genetically monogamous [28]), and that males also participate in social displays and all aspects of communal brood care, a complete description of social group stability would also include patterns of male transitions among breeding groups and potential fitness consequences of those transitions. Although we lack sufficient information to include males in our analyses of social bond duration, data from colour-banded adults collected in the early years of the project (n = 56 males and 66 females, 2006–2009), as well as genetic analyses of nestling relationships [28], indicate that male–female pairs often remain together for many years, and that they often join and leave breeding groups together. If mated pair-bonds are generally long-lasting, as these incomplete data suggest, it is possible that observed patterns of female associations are actually representative of both male and female associations. By contrast, if social groups are generally longer-lasting than pair bonds, transitions in membership may be more frequent for males than for females, and might have contributed to the unexplained variance in our dataset.
5. Conclusion
To our knowledge, this study is the first to show that the stability and duration of social affiliations have direct fitness consequences in a cooperatively breeding bird. This suggests that the affiliations themselves are shaped by natural selection, and are not simply by-products of kin structure [53] or fidelity to the group's territory or breeding site [54,55]. These results contribute to a growing body of evidence that social bonds have adaptive value in a wide range of group-living animals, and that the fitness consequences of these interactions must be considered to fully understand the evolution of stable social groups.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Chiti Arvind, Luke Carrabbia, Christa Morris, Laura Jara Reyes, Amanda G. Savagian and Zachariah Smart for assistance in the field. Dorothy Cheney, Andrew Gersick, Egbert G. Leigh, Jr., Daniel I. Rubenstein and Robert Seyfarth provided valuable feedback on the construction of the study and the analysis of the results. We are very grateful to the Smithsonian Tropical Research Institute for hosting this long-term project and providing infrastructure and support.
Ethics
The research in this study was approved by the Institutional Animal Care and Use Committee of the Smithsonian Tropical Research Institute. Genetic samples were collected and exported with approval from the Ministerio de Ambiente de Panamá, and imported to the USA with approval from the US Department of Agriculture.
Data accessibility
The data generated and analysed during this study is available in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.46tj577 [56]).
Authors' contributions
C.R. conceived the study and conducted statistical analyses. C.R. and M.J.S. collected data and conducted genetic analyses. C.R. wrote the paper with input from M.J.S. Both authors contributed to interpretation of data and presentation of results.
Competing interests
The authors declare no competing interests.
Funding
The long-term ani field monitoring programme was funded by the Smithsonian Tropical Research Institute, Princeton University and the Princeton Environmental Institute at Princeton University.
References
- 1.Massen JJ, Sterck EH, De Vos H. 2010. Close social associations in animals and humans: functions and mechanisms of friendship. Behaviour 147, 1379–1412. ( 10.1163/000579510X528224) [DOI] [Google Scholar]
- 2.Heathcote RJ, Darden SK, Franks DW, Ramnarine IW, Croft DP. 2017. Fear of predation drives stable and differentiated social relationships in guppies. Sci. Rep. 7, 41679 ( 10.1038/srep41679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104. ( 10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frère CH, Krützen M, Mann J, Connor RC, Bejder L, Sherwin WB. 2010. Social and genetic interactions drive fitness variation in a free-living dolphin population. Proc. Natl Acad. Sci. USA 107, 19 949–19 954. ( 10.1073/pnas.1007997107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanton MA, Mann J. 2012. Early social networks predict survival in wild bottlenose dolphins. PLoS ONE 7 e47508 ( 10.1371/journal.pone.0047508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter GG, Wilkinson GS. 2013. Food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Proc. R. Soc. B 280, 20122573 ( 10.1098/rspb.2012.2573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853. ( 10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silk JB. 2014. Evolutionary perspectives on the links between close social bonds, health, and fitness. In Sociality, hierarchy, health: comparative biodemography: a collection of papers (eds Weinstein M, Lane MA), pp. 121–144. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 9.Gero S, Engelhaupt D, Rendell L, Whitehead H. 2009. Who cares? Between-group variation in alloparental caregiving in sperm whales. Behav. Ecol. 20, 838–843. ( 10.1093/beheco/arp068) [DOI] [Google Scholar]
- 10.Ebensperger LA, Correa LA, León C, Ramírez-Estrada J, Abades S, Villegas Á, Hayes LD. 2016. The modulating role of group stability on fitness effects of group size is different in females and males of a communally rearing rodent. J. Anim. Ecol. 85, 1502–1515. ( 10.1111/1365-2656.12566) [DOI] [PubMed] [Google Scholar]
- 11.Gazda SK, Connor RC, Edgar RK, Cox F. 2005. A division of labour with role specialization in group-hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida. Proc. R. Soc. B 272, 135–140. ( 10.1098/rspb.2004.2937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilby IC, Machanda ZP, Mjungu DC, Rosen J, Muller MN, Pusey AE, Wrangham RW. 2015. ‘Impact hunters’ catalyse cooperative hunting in two wild chimpanzee communities. Phil. Trans. R. Soc. B 370, 20150005 ( 10.1098/rstb.2015.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Port M, Kappeler PM, Johnstone RA. 2011. Communal defense of territories and the evolution of sociality. Am. Nat. 178, 787–800. ( 10.1086/662672) [DOI] [PubMed] [Google Scholar]
- 14.Jaeggi AV, Van Schaik CP. 2011. The evolution of food sharing in primates. Behav. Ecol. Sociobiol. 65, 2125–2140. ( 10.1007/s00265-011-1221-3) [DOI] [Google Scholar]
- 15.Carter GG, Wilkinson GS. 2015. Social benefits of non-kin food sharing by female vampire bats. Proc. R. Soc. B 282, 20152524 ( 10.1098/rspb.2015.2524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis K, Belliveau M, Herndon B, Keller J. 2007. Group cognition, membership change, and performance: investigating the benefits and detriments of collective knowledge. Org. Behav. Hum. Decis. Processes 103, 159–178. ( 10.1016/j.obhdp.2007.01.005) [DOI] [Google Scholar]
- 17.McCarter MW, Sheremeta RM. 2013. You can't put old wine in new bottles: the effect of newcomers on coordination in groups. PLoS ONE 8, e55058 ( 10.1371/journal.pone.0055058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Möller LM, Beheregaray LB, Harcourt RG, Krützen M. 2001. Alliance membership and kinship in wild male bottlenose dolphins (Tursiops aduncus) of southeastern Australia. Proc. R. Soc. Lond. B 268, 1941–1947. ( 10.1098/rspb.2001.1756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silk JB, Altmann J, Alberts SC. 2006. Social relationships among adult female baboons (Papio cynocephalus). I. Variation in the strength of social bonds. Behav. Ecol. Sociobiol. 61, 183–195. ( 10.1007/s00265-006-0249-2) [DOI] [Google Scholar]
- 20.Mitani JC. 2009. Male chimpanzees form enduring and equitable social bonds. Anim. Behav. 77, 633–640. ( 10.1016/j.anbehav.2008.11.021) [DOI] [Google Scholar]
- 21.Smith JE, Van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE. 2010. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 284–303. ( 10.1093/beheco/arp181) [DOI] [Google Scholar]
- 22.Carter KD, Seddon JM, Frère CH, Carter JK, Goldizen AW. 2013. Fission–fusion dynamics in wild giraffes may be driven by kinship, spatial overlap and individual social preferences. Anim. Behav. 85, 385–394. ( 10.1016/j.anbehav.2012.11.011) [DOI] [Google Scholar]
- 23.Podgórski T, Lusseau D, Scandura M, Sönnichsen L, Jędrzejewska B. 2014. Long-lasting, kin-directed female interactions in a spatially structured wild boar social network . PLoS ONE 9, e99875 ( 10.1371/journal.pone.0099875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Waal FB. 1986. The integration of dominance and social bonding in primates. Q. Rev. Biol. 61, 459–479. ( 10.1086/415144) [DOI] [PubMed] [Google Scholar]
- 25.Silk JB. 2007. Social components of fitness in primate groups. Science 317, 1347–1351. ( 10.1126/science.1140734) [DOI] [PubMed] [Google Scholar]
- 26.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 27.Riehl C, Jara L. 2009. Natural history and reproductive biology of the communally breeding greater ani (Crotophaga major) at Gatún Lake, Panama. Wilson J. Ornithol. 121, 679–687. ( 10.1676/09-017.1) [DOI] [Google Scholar]
- 28.Riehl C. 2012. Mating system and reproductive skew in a communally breeding cuckoo: hard-working males do not sire more young. Anim. Behav. 84, 707–714. ( 10.1016/j.anbehav.2012.06.028) [DOI] [Google Scholar]
- 29.Riehl C, Strong MJ, Edwards SV. 2015. Inferential reasoning and egg rejection in a cooperatively breeding cuckoo. Anim. Cogn. 18, 75–82. ( 10.1007/s10071-014-0778-4) [DOI] [PubMed] [Google Scholar]
- 30.Riehl C, Strong MJ. 2015. Social living without kin discrimination: experimental evidence from a communally breeding bird. Behav. Ecol. Sociobiol. 69, 1293–1299. ( 10.1007/s00265-015-1942-9) [DOI] [Google Scholar]
- 31.Riehl C. 2010. Living with strangers: direct benefits favour non-kin cooperation in a communally nesting bird. Proc. R. Soc. B 278, 1728–1735. ( 10.1098/rspb.2010.1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strong MJ, Sherman BL, Riehl C. In press Home field advantage, not group size, predicts outcomes of intergroup conflicts in a social bird. Anim. Behav. ( 10.1016/j.anbehav.2017.07.006) [DOI] [Google Scholar]
- 33.Lau P, Bosque C, Strahl SD. 1998. Nest predation in relation to nest placement in the greater ani (Crotophaga major). Ornitol. Neotrop. 9, 87–92. [Google Scholar]
- 34.Vehrencamp SL. 1977. Relative fecundity and parental effort in communally nesting anis, Crotophaga sulcirostris. Science 197, 403–405. ( 10.1126/science.197.4301.403) [DOI] [PubMed] [Google Scholar]
- 35.Vehrencamp SL. 1978. The adaptive significance of communal nesting in groove-billed anis (Crotophaga sulcirostris). Behav. Ecol. Sociobiol. 4, 1–33. ( 10.1007/BF00302558) [DOI] [Google Scholar]
- 36.Vehrencamp SL, Bowen BS, Koford RR. 1986. Breeding roles and pairing patterns within communal groups of groove-billed anis. Anim. Behav. 34, 347–366. ( 10.1016/S0003-3472(86)80103-8) [DOI] [Google Scholar]
- 37.Riehl C. 2010. Egg ejection risk and hatching asynchrony predict egg mass in a communally breeding cuckoo, the greater ani (Crotophaga major). Behav. Ecol. 21, 676–683. ( 10.1093/beheco/arq038) [DOI] [Google Scholar]
- 38.Schmaltz G, Somers CM, Sharma P, Quinn JS. 2006. Non-destructive sampling of maternal DNA from the external shell of bird eggs. Conserv. Genet. 7, 543–549. ( 10.1007/s10592-005-9065-x) [DOI] [Google Scholar]
- 39.Riehl C. 2010. A simple rule reduces costs of extragroup parasitism in a communally breeding bird. Curr. Biol. 20, 1830–1833. ( 10.1016/j.cub.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 40.Almany GR, et al. 2009. Permanent genetic resources added to molecular ecology resources database 1 May 2009–31 July 2009. Mol. Ecol. Resour. 9, 1460–1466. ( 10.1111/j.1755-0998.2009.02759.x) [DOI] [PubMed] [Google Scholar]
- 41.Burnham KP, Anderson DR. 2003. Model selection and multimodel inference: a practical information-theoretic approach. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 42.Arnold TW. 2010. Uninformative parameters and model selection using Akaike's information criterion. J. Wildl. Manage. 74, 1175–1178. ( 10.1111/j.1937-2817.2010.tb01236.x) [DOI] [Google Scholar]
- 43.Shaffer TL. 2004. A unified approach to analyzing nest success. Auk 121, 526–540. ( 10.1642/0004-8038(2004)121%5B0526:AUATAN%5D2.0.CO;2) [DOI] [Google Scholar]
- 44.Porter TA, Wilkinson GS. 2001. Birth synchrony in greater spear-nosed bats (Phyllostomus hastatus). J. Zool. 253, 383–390. ( 10.1017/S0952836901000358) [DOI] [Google Scholar]
- 45.Koenig B. 1994. Components of lifetime reproductive success in communally and solitarily nursing house mice—a laboratory study. Behav. Ecol. Sociobiol. 34, 275–283. ( 10.1007/BF00183478) [DOI] [Google Scholar]
- 46.Lambin X. 1993. Determinants of the synchrony of reproduction in Townsend's voles, Microtus townsendii. Oikos 1, 107–113. ( 10.2307/3545101) [DOI] [Google Scholar]
- 47.Stern K, McClintock MK. 1998. Regulation of ovulation by human pheromones. Nature 392, 177–179. ( 10.1038/32408) [DOI] [PubMed] [Google Scholar]
- 48.Schank JC. 2001. Menstrual-cycle synchrony: problems and new directions for research. J. Comp. Psychol. 115, 3–15. ( 10.1037/0735-7036.115.1.3) [DOI] [PubMed] [Google Scholar]
- 49.Petrulis A. 2013. Chemosignals, hormones and mammalian reproduction. Horm. Behav. 63, 723–741. ( 10.1016/j.yhbeh.2013.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagelin JC, Jones IL. 2007. Bird odors and other chemical substances: a defense mechanism or overlooked mode of intraspecific communication? Auk 124, 741–761. ( 10.1642/0004-8038(2007)124%5B741:BOAOCS%5D2.0.CO;2) [DOI] [Google Scholar]
- 51.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 52.Ball GF. 1993. The neural integration of environmental information by seasonally breeding birds. Am. Zool. 33, 185–199. ( 10.1093/icb/33.2.185) [DOI] [Google Scholar]
- 53.Chapple DG, Scott Keogh J. 2006. Group structure and stability in social aggregations of White's skink, Egernia whitii. Ethol 112, 247–257. ( 10.1111/j.1439-0310.2006.01153.x) [DOI] [Google Scholar]
- 54.Godard R. 1991. Long-term memory of individual neighbours in a migratory songbird. Nature 350, 228–229. ( 10.1038/350228a0) [DOI] [Google Scholar]
- 55.Shizuka D, Chaine AS, Anderson J, Johnson O, Laursen IM, Lyon BE. 2014. Across-year social stability shapes network structure in wintering migrant sparrows. Ecol. Lett. 17, 998–1007. ( 10.1111/ele.12304) [DOI] [PubMed] [Google Scholar]
- 56.Riehl C, Strong MJ. 2018. Stable social relationships between unrelated females increase individual fitness in a cooperative bird Dryad Digital Repository. ( 10.5061/dryad.46tj577) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Riehl C, Strong MJ. 2018. Stable social relationships between unrelated females increase individual fitness in a cooperative bird Dryad Digital Repository. ( 10.5061/dryad.46tj577) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data generated and analysed during this study is available in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.46tj577 [56]).