Summary
Belatacept is a second‐generation cytotoxic T lymphocyte antigen (CTLA)‐4 immunoglobulin (Ig) fusion protein approved for immunosuppression in renal transplant recipients. It was designed intentionally to interrupt co‐stimulation via CD28 by binding to its ligands B7·1 and B7·2. Experimental evidence suggests a potential additional mechanism for CTLA‐4 Ig compounds through binding to B7 molecules expressed on antigen‐presenting cells (APCs) and up‐regulation of indoleamine 2,3‐dioxygenase (IDO), an immunomodulating enzyme that catalyzes the degradation of tryptophan to kynurenine and that down‐regulates T cell immunity. So far it remains unknown whether belatacept up‐regulates IDO in transplant recipients. We therefore investigated whether belatacept therapy enhances IDO activity in liver transplant recipients enrolled in a multi‐centre, investigator‐initiated substudy of the Phase II trial of belatacept in liver transplantation (IM103‐045). Tryptophan and kynurenine serum levels were measured during the first 6 weeks post‐transplant in liver transplant patients randomized to receive either belatacept or tacrolimus‐based immunosuppression. There was no significant difference in IDO activity, as indicated by the kynurenine/tryptophan ratio, between belatacept and tacrolimus‐treated patients in per‐protocol and in intent‐to‐treat analyses. Moreover, no evidence was found that belatacept affects IDO in human dendritic cells (DC) in vitro. These data provide evidence that belatacept is not associated with detectable IDO induction in the clinical transplant setting compared to tacrolimus‐treated patients.
Keywords: belatacept; indoleamine 2,3‐dioxygenase (IDO); liver transplantation; tryptophan metabolism
Introduction
Binding of CD28 on T cells to B7·1 (CD80) and B7·2 (CD86) on antigen‐presenting cells (APCs) is a crucial co‐stimulatory route to activate naive T cells 1. Activated conventional T cells and regulatory T cells (Tregs) express cytotoxic T lymphocyte antigen (CTLA)‐4, which counterbalances CD28 signals by competitively binding B7 molecules with a distinctly higher affinity 2. This physiological control mechanism provides the conceptual framework for the rational design of the fusion‐protein CTLA‐4 immunoglobulin (Ig) (abatacept), which is composed of the extracellular CTLA‐4 domain and a mutated Fc part of human IgG1 3. Belatacept is a second‐generation CTLA‐4 Ig approved for use in renal transplant recipients which differs from abatacept in two amino acids at the CTLA‐4 binding region to increase its avidity to B7·1 and B7·2 3. Belatacept seems to have advantages over cyclosporin in terms of renal function as well as patient/graft survival 4, 5, but is also associated with higher incidences of acute rejection episodes and post‐transplant lymphoproliferative disease (PTLD) 6.
The mechanisms of action of belatacept are incompletely understood, and might go well beyond simple blockade of CD28 7. Notably, it has been proposed that CTLA‐4 Ig (as well as endogenous CTLA‐4) up‐regulates indoleamine 2,3‐dioxygenase (IDO) (recently termed IDO‐1) via reverse signalling through B7·1/2 on APCs 8, 9. IDO is expressed preferentially by professional APCs and exhibits an immunomodulating function through catalyzing the degradation of the essential amino acid tryptophan to kynurenine. The local deprivation of tryptophan forces surrounding effector cells into cell‐cycle arrest, while emanating kynurenines can suppress T cell immunity 10. Regulating tryptophan metabolism is an innate mechanism of host defence against infections, but was found later to also sustain fetal tolerance and to inhibit local tissue inflammation 10. Several studies suggest a role of IDO for the preservation of transplanted organs in a CTLA‐4 Ig‐dependent manner. Long‐term survival of murine pancreatic islet allografts induced by CTLA‐4 Ig could be abrogated through treatment with the IDO inhibitor 1‐methyl‐tryptophan (1‐MT) 9. Furthermore, a critical impact of IDO together with regulatory T cells was described in murine heart transplant recipients treated with CTLA‐4 Ig and donor‐specific transfusion (but not CTLA‐4 Ig treatment alone) 11. Increased numbers of peripheral CD16+/IDO‐1+ blood cells were described under belatacept therapy 12. However, other studies did not find a critical role of IDO in a murine mixed chimerism transplantation model based on CTLA‐4 Ig 13 and only a marginal effect of human IDO‐producing DCs on alloreactivity 14. Further, abatacept was shown to inhibit T cell proliferation in vitro independently of IDO 15.
It remains undetermined whether belatacept induces IDO activity in transplant recipients. We therefore investigated the potential impact of belatacept on IDO activity in liver transplant patients treated with either belatacept or tacrolimus and tested whether belatacept induces IDO activity in vitro in human DCs.
Materials and methods
Patients
Patients who were participating in the controlled, randomized Phase II multi‐centre trial IM103‐045 ‘Evaluation of belatacept as First‐Line Immunosuppression in De‐Novo Liver Transplant Recipients’ 16 were enrolled upon written informed consent in this prospective, investigator‐initiated substudy performed at four transplant centres (Medical University of Vienna, Vienna, Austria; University Hospital of Tübingen, Tübingen, Germany; Hanover Medical School, Hanover, Germany; Charité‐Campus Virchow Klinikum, Berlin, Germany). Patients were treated with either belatacept or tacrolimus as the main immunosuppression, together with prednisone, with or without mycophenolate mofetil, and with or without blinded administration of basiliximab induction therapy (for details of the five therapy arms in the core study protocol IM103‐045 see 16). Belatacept dosing was equal in all patients receiving belatacept during the observation period of 6 weeks (10 mg/kg on days 1, 3, 5, 14 and 28). The study was approved by the respective Institutional Review Board of each participating transplant centre and performed in accordance with the ethical standards laid down in the Declaration of Helsinki Principles.
Statistical analysis
The predefined primary end‐point was the ratio of kynurenine to tryptophan (kyn/trp) measured in serum 5, 14, 28 and 42 days after transplantation. Based on results from renal transplant recipients 17, the sample size was determined with the hypothesis that in liver transplant recipients treated with tacrolimus kyn/trp = 55 ± 40, and that in patients in whom IDO might be activated through belatacept kyn/trp = 114 ± 45. With an assumed dropout rate of 30% and an alpha error of 0·05, it was estimated that 30 patients need to be enrolled for the study to have at least 80% power to detect a significant difference between tacrolimus‐ and belatacept‐treated patients on a per‐protocol analysis.
Time and group effects of the in‐vivo studies were analysed employing a mixed linear regression model. Intragroup comparisons pretransplant and on day 5 after transplantation were performed using the paired t‐test; χ2 and t‐tests were used to compare patient characteristics between treatment groups. Statistical analyses of in‐vitro studies were performed using the t‐test. A P‐value below 0·05 was considered as statistically significant. Analyses were performed with sas version 9·4 for Windows (Cary, NC, USA) or Microsoft Excel.
Blood sampling
Blood samples were collected immediately before and 5, 14, 28 and 42 days after transplantation, before administration of the study drug. Blood samples were centrifuged and serum was stored at −80° C until shipment on dry ice to the analysis laboratory (Biocentre, Innsbruck, Austria) and analysed in batches.
IDO activity measurement in patient serum and in supernatant in vitro
Tryptophan and kynurenine concentrations in human serum were determined by reverse‐phase high‐pressure liquid chromatography (HPLC). Specimens were deproteinized with trichloroacetic acid and were separated on reverse‐phase C18 material using 0·015 M potassium phosphate buffer (pH = 6·4). Tryptophan was monitored by means of its native fluorescence at 285 nm excitation and 360 nm emission wavelengths; kynurenine was detected by ultraviolet (UV)‐absorption at 365 nm wavelength in the same chromatographic run. Finally, kyn/trp was calculated as an indirect estimate of IDO activity by dividing kynurenine concentrations (µmol/l) by tryptophan concentrations (mmol/l).
Samples for in‐vitro studies on human DCs
All in‐vitro experiments were performed using human blood obtained from healthy volunteers or from blood donors at the blood bank of the Vienna General Hospital upon giving informed consent.
Cell isolation and in‐vitro treatment
Peripheral blood mononuclear cells (PBMCs) were enriched from whole blood by density centrifugation (Lymphoprep, Nycomed, Oslo, Norway). Monocyte enrichment was performed via counterflow centrifugal elutriation (Elutra Cell Separation System, Gambro BCT, Lakewood, CA, USA) 18 resulting in a > 85% CD14+ population. This monocyte‐enriched cell population was plated in culture flasks (Iwaki, Sterilin, Aberbargoed, UK) at a density of 0·3–0·5 × 106 cells/cm2. Immature DCs (iDCs) were generated by culture in complete medium supplemented with 1000 U/ml granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (CellGenix, Freiburg, Germany) and 400 U/ml IL‐4 (CellGenix) for 5 days. iDCs were stimulated through exposure to lipopolysaccharide (LPS) (50 ng/ml) (Calbiochem, La Jolla, CA, USA; Escherichia coli O111:B4) for 24 h and treated subsequently with or without graded doses (20 µg/ml, 100 µg/ml or 200 µg/ml) of belatacept for another 24 h. The quality of DC generation was monitored by visual and flow cytometric evaluation of a typical DC morphology and expression of cell surface markers, respectively, with purity typically being > 80%, as described earlier 19 (Fig. 1). Highly enriched T cell populations were prepared as described 20. In brief, CD3+ T cells were isolated from the manufacturer's instructions, resulting typically in a > 95% enrichment of the targeted cell population. Purity and viability were monitored by flow cytometry.
Figure 1.
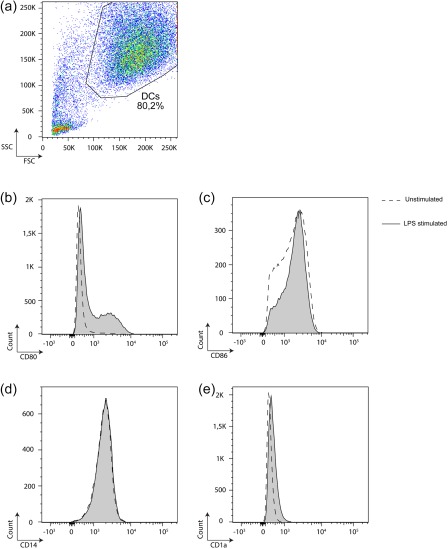
Purity of immature and stimulated dendritic cells (DCs) with granulocyte–macrophage colony‐stimulatory factor (GM‐CSF) and interleukin (IL)‐4 for 5 days. Immature DCs (iDCs) were stimulated through exposure to lipopolysaccharide (LPS) for 24 h. Quality and purity of DC generation was monitored by visual and flow cytometric evaluation of a typical DC morphology (a) and expression of cell surface markers CD80, CD86, CD14 and CD1a (b–e), respectively. A representative analysis is shown. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Cell culture medium
Dendritic cell (DC) differentiation and stimulation was performed in AIM V cell culture medium (Gibco, Invitrogen, Carlsbad, CA, USA) containing 2% human plasma (Octaplas, Octapharm, Vienna, Austria) and 1% v/v L‐glutamine (PAA Laboratories, Pasching, Austria), hereafter termed complete medium. T cell stimulation was performed in complete medium supplemented with 25 mM HEPES (PAA Laboratories). All cell cultures were maintained in humidified air containing 5% CO2 at 37°C.
Flow cytometry
The following monoclonal antibodies (mAb) were used: isotype control, IgG1‐ fluorescein isothiocyanate (FITC), IgG2a‐phycoerythrin (PE), IgG1‐PE, IgG2a‐peridinin chlorophyll (perCP), IgG1‐allophycocyanin; anti‐CD45‐perCP (clone 2D1), anti‐CD3‐allophycocyanin (clone SK7), anti‐CD25‐PE (clone 2A3), anti‐CD14‐perCP‐cyanin5·5 (Cy5·5) (clone M5E2) (all from BD Biosciences, San Jose, CA, USA); anti‐human leucocyte antigen (HLA) class II‐FITC (clone CR3/43) and anti‐HLA class I‐PE (clone W6/32) (Dako, Vienna, Austria); a fluorescence activator cell sorter (FACS)Calibur and a FACS‐LSR2 flow cytometer (BD BioSciences) were used for acquisition and FlowJo (Tree Star, Inc., Ashland, OR, USA) software was used for analysis of list mode data.
T cell stimulation and mixed lymphocyte reaction (MLR)
Highly enriched total CD3+ T cells were co‐cultured with differently stimulated allogeneic DCs (DC : T cell ratio 1 : 10) for 7 days in 24‐well plates (Iwaki) in the presence or absence of belatacept (100 µg/ml), as indicated. T cells were stained with carboxyfluorescein succinimidyl ester (CFSE) and proliferation was calculated on day 7 as the percentage of CFSE‐negative cells.
IDO expression in DCs
IDO protein expression in DCs was investigated by immunoblot analysis with a mouse monoclonal anti‐human IDO antibody, kindly provided by O. Takikawa 21.
Results
IDO activity in de‐novo liver transplant recipients is not increased under belatacept‐based immunosuppression
A prospective, investigator‐driven substudy of the Phase II multi‐centre trial IM103‐045 of belatacept in de‐novo liver transplantation was performed. Thirty patients were enrolled into the substudy, who were treated either with belatacept‐based (n = 23) or tacrolimus‐based immunosuppression (n = 7), according to the trial protocol of the IM103‐045 study 16. Kynurenine and tryptophan levels as well as kyn/trp as an indicator of IDO activity were measured in serum during the first 6 weeks (on days 5, 14, 28 and 42) post‐liver transplantation. During this immediate post‐transplant period belatacept dosing was at its peak, and the dosing schedule was the same in the three belatacept arms of the parent trial. The kyn/trp ratio served as an established indicator of IDO activity 9, 17. Nine of 23 patients treated with belatacept and one of seven patients treated with tacrolimus discontinued the parent study before day 42, so that 14 and six patients, respectively, were included in the per‐protocol analysis that was prespecified in the study design. Demographic characteristics were comparable between the two analysed treatment groups (Table 1).
Table 1.
Demographic characteristics of liver transplant recipients by treatment group (per protocol analysis)
| Belatacept (n = 14) | Tacrolimus (n = 6) | P‐value | |
|---|---|---|---|
| Recipient male sex (%) | 64 | 66 | 0·32 |
| Mean age (years) | 51·7 | 58·3 | 0·29 |
| Mean MELD score at time of transplantation; mean | 18·9 | 16·7 | 0·55 |
| Hepatitis C infection (%) | 28·6 | 16·7 | 0·57 |
| Body mass index; mean | 26·8 | 26·2 | 0·67 |
MELD = model for end‐stage liver disease.
Statistical analysis calculated by a mixed linear model showed no significant change in the time–course of tryptophan, kynurenin and the computed ratio kynurenine/tryptophan for belatacept and tacrolimus‐treated patients (Table 2). In detail, tryptophan levels were similar pretransplant in patients treated with belatacept or tacrolimus [belatacept: 59·55 μmol/l ± 14·43 versus tacrolimus: 61·73 μmol/l ± 31·06, P = 0·89; mean values ± standard deviation (s.d.)], decreased in both groups in the early (day 5) post‐transplant period (belatacept: 43·89 μmol/l ± 15·97, P < 0·05 versus pretransplant; tacrolimus: 34·47 μmol/l ± 15·15, P = 0·09 versus pretransplant), and no evidence of a significant difference was observed between the two treatment groups throughout the follow‐up [P = 0·98 (day 14), P = 0·61 (day 28), P = 0·74 (day 42) Fig. 2a]. Kynurenine levels, in contrast, changed little by 5 days after transplantation (belatacept: 2·57 μmol/l ± 1·17 versus 2·36 μmol/l ± 1·21, P = 0·67; tacrolimus: 2·36 μmol/l ± 0·77 versus 2·22 μmol/l ± 1·04, P = 0·81). Again, no evidence of a significant difference of kynurenine levels between both treatment groups was observed during the subsequent observation period [P = 0·38 (day 14), P = 0·13 (day 28), P = 0·51 (day 42); Fig. 2b]. IDO activity, estimated via kynurenine/tryptophan ratio was increased numerically, but not significantly, in both treatment groups immediately after transplantation [belatacept: 63·89 μmol/mmol ± 55·88 (day 5) versus 43·35 μmol/mmol ± 16·82 (pretransplant), P = 0·24; tacrolimus: 71·75 μmol/mmol ± 26·64 (day 5) versus 42·87 μmol/mmol ± 15·88 (pretransplant), P = 0·06; Fig. 2c]. In addition, an intent‐to‐treat analysis was performed post‐hoc (n = 23 belatacept, n = 7 tacrolimus), which again did not reveal a detectable difference in IDO activity between belatacept‐ and tacrolimus‐treated patients [P = 0·43 (day 0), P = 0·45 (day 5), P = 0·63 (day 14), P = 0·59 (day 28), P = 0·54 (day 42), Fig. 2d–f]. Neopterin levels, measured as control parameter, underwent no significant increase or decrease during the whole observation period (not shown) arguing against a significant general activation of the immune system after liver transplantation 22.
Table 2.
Estimates for the mixed linear model
| Effect | Estimate | Lower 95% CL* | Upper 95% CL | P‐value |
|---|---|---|---|---|
| Tryptophan | ||||
| Intercept (µmol/l) | 52·59 | 40·04 | 65·15 | <·001 |
| Therapy (bel † versus tac ‡ ) (µmol/l) | −1·10 | −15·47 | 13·26 | 0·873 |
| Days (µmol/l day) | 0·16 | −0·32 | 0·63 | 0·512 |
| Days × therapy (bel versus tac) (µmol/l day) | −0·13 | −0·68 | 0·41 | 0·632 |
| MMF (yes versus no) | 12·25 | −2·93 | 27·42 | 0·107 |
| Kynurenine | ||||
| Intercept (µmol/l) | 2·69 | 1·83 | 3·55 | < 0·001 |
| Therapy (bel versus tac) (µmol/l) | −0·24 | −1·23 | 0·75 | 0·613 |
| Days (µmol/L day) | 0·00 | −0·03 | 0·03 | 0·960 |
| Days × therapy (bel versus tac) (µmol/l day) | −0·01 | −0·04 | 0·02 | 0·471 |
| MMF (yes versus no) | 0·61 | −0·54 | 1·76 | 0·279 |
| Kynurenine/tryptophan ratio | ||||
| Intercept (µmol/l) | 55·78 | 34·95 | 76·61 | < 0·001 |
| Therapy (bel versus tac) (µmol/l) | −4·95 | −28·84 | 18·94 | 0·668 |
| Days (µmol/l day) | −0·25 | −1·00 | 0·51 | 0·519 |
| Days × therapy (bel versus tac) (µmol/l day) | 0·02 | −0·86 | 0·89 | 0·969 |
| MMF (yes versus no) | 0 | −26·39 | 22·98 | 0·886 |
*Confidence limit; †belatacept; ‡tacrolimus. MMF = mycophenolate mofetil.
Figure 2.
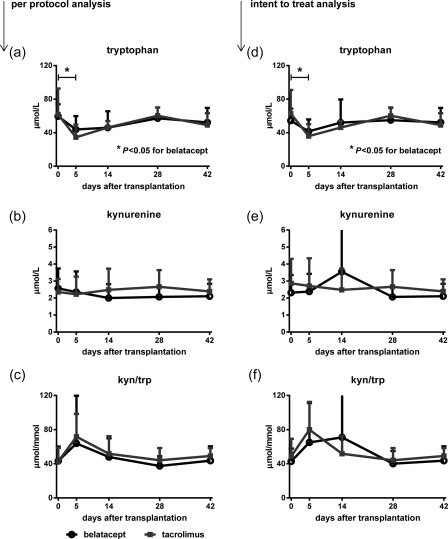
Belatacept does not induce indoleamine 2,3‐dioxygenase (IDO) activity in liver transplant patients. Two groups of liver transplant recipients were compared with a per‐protocol analysis depending on whether they received belatacept‐based (n = 14) or tacrolimus‐based (n = 6) immunosuppression. (a) Tryptophan levels measured in serum were similar pretransplant in patients treated with belatacept or tacrolimus, decreased in both groups in the early post‐transplant period (pretransplant versus d5: *P < 0·05 for belatacept) and were comparable between both groups throughout follow‐up [P = not significant (n.s.)]. (b) Kynurenine levels were comparable over time between both groups. (c) The calculated kynurenine/tryptophan ratio, an indicator of IDO‐activity, was comparable between patients treated with belatacept or tacrolimus during the first 6 weeks post‐liver transplantation (P = n.s. for all combinations). (d–f) A post‐hoc intent‐to‐treat analysis of all enrolled patients showed similar results as the per‐protocol analysis, with comparable IDO activity in patients treated with belatacept and tacrolimus. Mean values and standard deviation (error bars) are shown (a–f).
The kynurenine/tryptophan ratio was not significantly different between patients with tacrolimus versus patients with belatacept throughout the whole observation period (Fig. 2c). Taken together, these results provide evidence that IDO activation is not a mechanism of action of belatacept‐based immunosuppression in organ transplantation.
Impact of belatacept on tryptophan catabolism in human monocyte‐derived DCs
Several studies imply that reverse signalling triggered through CTLA‐4 Ig ligating B7 on DCs induces tryptophan catabolism in vitro 9, but so far it remains unclear whether belatacept (a second‐generation CTLA‐4 Ig compound) can exert a similar function. Therefore, we also investigated the impact of belatacept on tryptophan catabolism in vitro, using human DCs. iDCs were either left unstimulated or were stimulated with LPS for 24 h. After extensive washing, DCs were treated with or without graded doses of belatacept. Treatment of unstimulated DCs with increasing doses of belatacept did not induce IDO protein expression. Activation of iDCs with LPS led to slight IDO expression, as described previously 19, which was not increased after subsequent treatment even with high doses of belatacept (Fig. 3a). In a next step we evaluated IDO‐dependent tryptophan catabolism. iDCs were stimulated as described above and recultured in fresh medium for another 48 h before analysing supernatants for kynurenine accumulation. Unstimulated DCs did not release kynurenine, even when incubated with increasing doses of belatacept (Fig. 3b). In contrast, LPS prestimulation induced kynurenine release, but which was not increased after subsequent treatment with ascending amounts of belatacept (Fig. 3b). These observations suggest that belatacept does not lead to significant induction or enhancement of IDO activity in human DCs in vitro.
Figure 3.
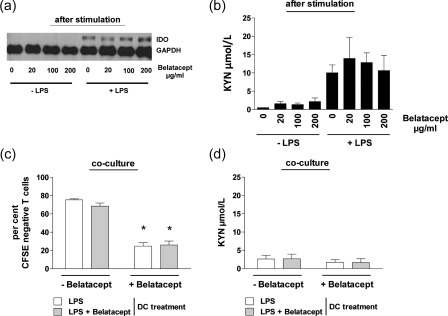
Belatacept does not induce indoleamine 2,3‐dioxygenase (IDO) activity in vitro in human monocyte‐derived dendritic cells (DCs). (a) Immature DCs (iDCs) were either left unstimulated or were stimulated with lipopolysaccharide (LPS) (50 ng/ml) for 24 h and treated subsequently with or without graded doses of belatacept (20 µg/ml, 100 µg/ml, 200 µg/ml). Treatment of unstimulated DCs with increasing doses of belatacept did not induce IDO protein expression. Activation of iDCs with LPS (50 ng/ml) resulted in IDO protein expression which was, however, not increased in the presence of belatacept. (b) DCs were recovered and recultured in fresh medium for another 48 h. Supernatants were analysed for kynurenine accumulation after stimulation and after reculture. DCs without LPS prestimulation did not release kynurenine independent of further belatacept treatment. LPS prestimulation induced kynurenine release, which was not increased upon addition of increasing doses of belatacept. (c) Differently stimulated DCs were harvested and co‐cultured in the presence of allogeneic T cells (DC : T cell ratio 1 : 10) for 7 days, each condition in the presence or absence of belatacept (100 µg/ml). Neither LPS‐stimulated nor LPS/belatacept‐stimulated DCs dampened proliferation of CD3+ T cells (mean proliferation 75 and 66%, respectively, P = not significant). In contrast, the co‐culture of both DC populations and CD3+ T cells in the presence of belatacept resulted in a significantly lower proliferative response (mean proliferation 25 and 26%, respectively, *P < 0·001). (d) Kynurenine release was equally low during the first 48 h of co‐culture, independent of DC‐type or presence or absence of belatacept.
We next investigated the effect of belatacept in a human MLR. iDCs were stimulated with LPS for 24 h before LPS washout and treatment with or without belatacept for another 24 h. Differently stimulated DCs were harvested and co‐cultured in the presence of allogeneic T cells for 7 days, each condition in the presence or absence of belatacept. T cell proliferation was not inhibited after co‐culture with LPS‐stimulated DCs irrespective of previous pretreatment with/without belatacept (proliferation as indicated by CFSE‐negative cells: 75% without belatacept versus 66% with belatacept, P = not significant). As expected, the co‐culture of both DC populations and CD3+ T cells in the presence of belatacept resulted in a significantly lower proliferative response (mean proliferation 25 and 26%, respectively) (P < 0·001) (Fig. 3c). To investigate whether this effect was associated with increased IDO activity under belatacept treatment we analysed kynurenine accumulation in either co‐culture condition. Kynurenine release was comparably low during the first 48 h of co‐culture, independent of DC‐type or presence/absence of belatacept (Fig. 3d). Taken together, these results demonstrate that belatacept does not induce IDO activity in human monocyte‐derived DCs.
Discussion
In this study we found no evidence that belatacept increases IDO activity when given as immunosuppressive therapy in liver transplant recipients or when used to stimulate human DC in vitro.
Due to the gradual realization of the complexity of the CD28 pathway, the mechanism(s) of action of anti‐B7 agents such as belatacept have again come under scrutiny. Designed originally with the intention to prevent activation of CD28 through saturation of its ligands B7·1/2, more recently the question arose as to whether CTLA‐4 Ig (abatacept, belatacept) could interfere with the physiological function of the CD28‐homologue CTLA‐4 that binds the same B7 ligands. This could, hypothetically, involve the blockade of a ‘beneficial’ inhibitory function of CTLA‐4 on Tregs, or alternatively the triggering of the B7‐dependent immunomodulatory function of IDO in APCs 3. While recent evidence suggests that interference with the CTLA‐4 function on Tregs depends upon the CTLA‐4 Ig dose 23, 24, the study presented herein addressed the question whether IDO is triggered as immunomodulatory mechanism in APCs by belatacept.
IDO activity was assessed by measuring serum levels of tryptophan and kynurenine whose ratio is indicative of intracellular IDO activity 10. This approach has already been employed to assess IDO activity in other settings such as autoimmune disease and cancer, demonstrating that the kyn/trp ratio is a clinically relevant parameter of IDO activity 10, 25, 26. By enrolling patients who participated in the Phase II multi‐centre trial of belatacept in liver transplantation, a randomized patient population could be investigated prospectively. No difference in the ratio of kynurenine to tryptophan was detectable between belatacept‐ and tacrolimus‐treated patients.
The high dropout rate might be a concern. We therefore compared the available values of dropout patients with patients of the per‐protocol analysis. The values of dropout patients were in the range of the per‐protocol analysed patients (data not shown). Study discontinuation was not associated with abnormal values of tryptophan and kynurenine analysed in this study. Importantly, an intent‐to‐treat analysis was also performed post‐hoc including all enrolled patients, which again did not reveal a detectable difference in IDO activity between belatacept‐ and tacrolimus‐treated patients, obviating the concern that the dropout rate is a source of significant bias.
Notably, tryptophan levels decreased (without accompanying increase in kynurenine) in both groups shortly after transplantation and returned to baseline levels in equal measure, which may be a result from the surgical procedure per se 27.
To rule out that belatacept triggered subtle effects in APCs which are locally restricted and not reflected in serum, we also investigated IDO activity in human DCs in vitro. iDCs were challenged with belatacept, selecting belatacept concentrations that go beyond the reported peak levels of belatacept in transplant recipients 28. Belatacept alone did not induce IDO activity in human monocyte‐derived DCs and did not enhance IDO activity further in DCs prestimulated with LPS (which induces per se moderate tryptophan metabolism in human DCs). Thus, we found no evidence that belatacept induces IDO activity in human DC, despite its higher affinity for B7·1/2. As expected, addition of belatacept to an MLR had a significant proliferation‐dampening effect, irrespective of which DCs were used as stimulators. This effect was independent of IDO activity, as kynurenine accumulation during these co‐cultures was equally low under each condition. It has been shown that Tregs, in contrast to conventional T cells, are resistant to CTLA‐4 Ig‐mediated inhibition of proliferation 29, which might contribute to the overall immunosuppressive effects of abatacept/belatacept.
Several factors could explain the difference between the current findings with belatacept and previous reports showing IDO induction with CTLA‐4 Ig. In general, the importance of the physiological role of ‘reverse’ signalling through B7 through ligation of CTLA‐4 remains controversial. The cytoplasmic tails of B7·1 and B7·2 are relatively short and do not contain common signalling motifs 2. Recent evidence points increasingly to a cell extrinsic mechanism of CTLA‐4 through physically removing B7·1/2 from APCs. Upon ligation by CTLA‐4, B7·1 and B7·2 are removed rapidly from the cell surface through trans‐endocytosis, leaving only a limited period of time for establishing a functional signalling cascade 2. Such a mechanism is not expected to be influenced by belatacept. Theoretically, belatacept's modified amino acid sequence in the binding region might influence its capacity to induce signalling via B7·1/2, although this appears unlikely. The in‐vitro studies suggesting that CTLA‐4 Ig can trigger a signal through B7·1/2 which leads to interferon (IFN)‐γ‐dependent induction of IDO in APCs 30 were obtained with ‘self‐made’ CTLA‐4 Ig fusion‐proteins that contained an unmodified IgG partly able to bind CD16 Fc receptors. In contrast, abatacept and belatacept contain a mutated/silenced Fc portion 31. Cross‐linking of CD16 by the dimeric fusion protein CTLA‐4 Ig could induce IFN‐γ expression which, in turn, would increase the activity of IDO. Importantly, to the best of our knowledge, the present study is the first to investigate belatacept's capacity to induce IDO.
In conclusion, these results indicate that the induction of the immunomodulatory enzyme IDO through reverse signalling via B7 is not a mechanism of action of the immunosuppressive effect of belatacept.
Author contributions
S. B. and B. J. designed and performed research, analysed and interpreted data and wrote the manuscript; T. W. designed research, analysed and interpreted data and wrote the manuscript; B. M. interpreted data and wrote the manuscript; A. Ka. analysed data; J. P., A. Ko., T. B., F. M., D. F. and G. B. performed research.
Disclosure
T. W. has received research support and honoraria from Bristol‐Myers Squibb. All other authors declare no conflicts of interest.
Acknowledgements
This work was supported by a research agreement with Bristol‐Myers Squibb (T. W.). The funder had no role in the design of this investigator‐initiated substudy, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Pilat N, Sayegh MH, Wekerle T. Costimulatory pathways in transplantation. Semin Immunol 2011; 23:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker LS, Sansom DM. The emerging role of CTLA4 as a cell‐extrinsic regulator of T cell responses. Nat Rev Immunol 2011; 11:852–63. [DOI] [PubMed] [Google Scholar]
- 3. Wekerle T, Grinyo JM. Belatacept: from rational design to clinical application. Transpl Int 2012; 25:139–50. [DOI] [PubMed] [Google Scholar]
- 4. Vincenti F, Rostaing L, Grinyo J et al Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med 2016; 374:333–43. [DOI] [PubMed] [Google Scholar]
- 5. Schwarz C, Mayerhoffer S, Berlakovich GA et al Long‐term outcome of belatacept therapy in de novo kidney transplant recipients – a case‐match analysis. Transpl Int 2015; 28:820–7. [DOI] [PubMed] [Google Scholar]
- 6. Snanoudj R, Tinel C, Legendre C. Immunological risks of minimization strategies. Transpl Int 2015; 28:901–10. [DOI] [PubMed] [Google Scholar]
- 7. Leibler C, Matignon M, Pilon C et al Kidney transplant recipients treated with belatacept exhibit increased naive and transitional B cells. Am J Transplant 2014; 14:1173–82. [DOI] [PubMed] [Google Scholar]
- 8. Fallarino F, Grohmann U, Hwang KW et al Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 2003; 4:1206–12. [DOI] [PubMed] [Google Scholar]
- 9. Grohmann U, Orabona C, Fallarino F et al CTLA‐4‐Ig regulates tryptophan catabolism in vivo . Nat Immunol 2002; 3:1097–101. [DOI] [PubMed] [Google Scholar]
- 10. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013; 34:137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sucher R, Fischler K, Oberhuber R et al IDO and regulatory T cell support are critical for cytotoxic T lymphocyte‐associated Ag‐4 Ig‐mediated long‐term solid organ allograft survival. J Immunol 2012; 188:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furuzawa‐Carballeda J, Lima G, Uribe‐Uribe N et al High levels of IDO‐expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplant Proc 2010; 42:3489–96. [DOI] [PubMed] [Google Scholar]
- 13. Pree I, Bigenzahn S, Fuchs D et al CTLA4Ig promotes the induction of hematopoietic chimerism and tolerance independently of indoleamine‐2,3‐dioxygenase. Transplantation 2007; 83:663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terness P, Chuang JJ, Bauer T, Jiga L, Opelz G. Regulation of human auto‐ and alloreactive T cells by indoleamine 2,3‐dioxygenase (IDO)‐producing dendritic cells: too much ado about IDO? Blood 2005; 105:2480–6. [DOI] [PubMed] [Google Scholar]
- 15. Davis PM, Nadler SG, Stetsko DK, Suchard SJ. Abatacept modulates human dendritic cell‐stimulated T‐cell proliferation and effector function independent of IDO induction. Clin Immunol 2008; 126:38–47. [DOI] [PubMed] [Google Scholar]
- 16. Klintmalm GB, Feng S, Lake JR et al Belatacept‐based immunosuppression in de novo liver transplant recipients: 1‐year experience from a phase II randomized study. Am J Transplant 2014; 14:1817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandacher G, Cakar F, Winkler C et al Non‐invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int 2007; 71:60–7. [DOI] [PubMed] [Google Scholar]
- 18. Dohnal AM, Graffi S, Witt V et al. Comparative evaluation of techniques for the manufacturing of dendritic cell‐based cancer vaccines. J Cell Mol Med 2009; 13(1):125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon‐gamma‐triggered indoleamine 2,3‐dioxygenase competence in human monocyte‐derived dendritic cells induces regulatory activity in allogeneic T cells. Blood 2009; 114:3235–43. [DOI] [PubMed] [Google Scholar]
- 20. Heitger A, Winklehner P, Obexer P et al Defective T‐helper cell function after T‐cell‐depleting therapy affecting naive and memory populations. Blood 2002; 99:4053–62. [DOI] [PubMed] [Google Scholar]
- 21. Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon‐gamma action – characterization of indoleamine 2,3‐dioxygenase in cultured human‐cells induced by interferon‐gamma and evaluation of the enzyme‐mediated tryptophan degradation in its anticellular activity. J Biol Chem 1988; 263:2041–8. [PubMed] [Google Scholar]
- 22. Fuchs D, Moller AA, Reibnegger G et al Increased endogenous interferon‐gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett 1991; 28:207–11. [DOI] [PubMed] [Google Scholar]
- 23. Vogel I, Verbinnen B, Van Gool S, Ceuppens JL. Regulatory T cell‐dependent and ‐independent mechanisms of immune suppression by CD28/B7 and CD40/CD40L costimulation blockade. J Immunol 2016; 197:533–40. [DOI] [PubMed] [Google Scholar]
- 24. Schwarz C, Unger L, Mahr B et al The immunosuppressive effect of CTLA4 immunoglobulin is dependent on regulatory T cells at low but not high doses. Am J Transplant 2016; 16:3404–15. [DOI] [PubMed] [Google Scholar]
- 25. Mancuso R, Hernis A, Agostini S et al Indoleamine 2,3 dioxygenase (IDO) Expression and activity in relapsing‐remitting multiple sclerosis. PLOS ONE 2015; 10:e0130715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Durr S, Kindler V. Implication of indolamine 2,3 dioxygenase in the tolerance toward fetuses, tumors, and allografts. J Leukoc Biol 2013; 93:681–7. [DOI] [PubMed] [Google Scholar]
- 27. Hol JW, Stolker RJ, Klimek M, Stronks DL, Fekkes D. The tryptophan kynurenine pathway, neopterin and IL‐6 during vulvectomy and abdominal hysterectomy. J Biomed Sci 2014; 21:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vincenti F, Blancho G, Durrbach A et al Five‐year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 2010; 21:1587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmadi SM, Holzl MA, Mayer E, Wekerle T, Heitger A. CTLA4‐Ig preserves thymus‐derived T regulatory cells. Transplantation 2014; 98:1158–64. [DOI] [PubMed] [Google Scholar]
- 30. Finger EB, Bluestone JA. When ligand becomes receptor–tolerance via B7 signaling on DCs. Nat Immunol 2002; 3:1056–7. [DOI] [PubMed] [Google Scholar]
- 31. Davis PM, Abraham R, Xu L, Nadler SG, Suchard SJ. Abatacept binds to the Fc receptor CD64 but does not mediate complement‐dependent cytotoxicity or antibody‐dependent cellular cytotoxicity. J Rheumatol 2007; 34:2204–10. [PubMed] [Google Scholar]


