Figure 2.
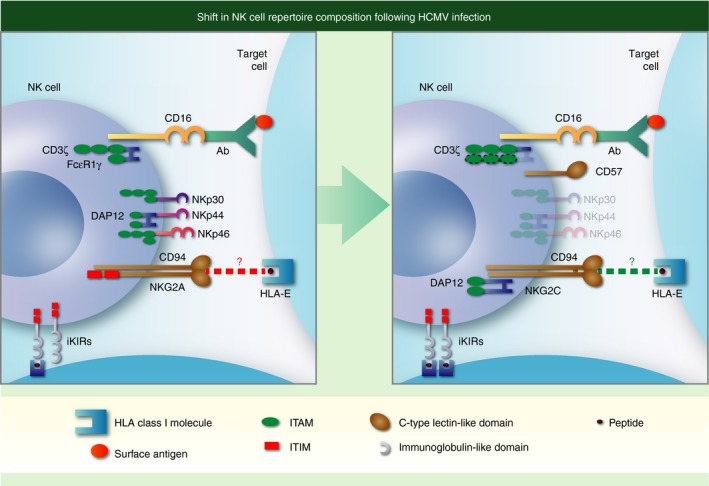
Adaptation of natural killer (NK) cells in response to human cytomegalovirus (HCMV) infection. Individuals infected with HCMV (right hand panel) have an increased fraction of circulating NK cells that express CD57 and the activating C‐type lectin‐like receptor NKG2C with reduced levels of natural cytotoxicity receptors NKp30, NKp44 and NKp46. These NK cells are skewed towards expression of activating KIR and self‐specific inhibitory killer cell immunoglobulin‐like receptors (iKIR) with cognate HLA‐I ligands present in the host, express higher levels of CD16 and are more effective at mediating antibody‐dependent cell‐mediated cytotoxicity. Enhanced triggering through CD16 is associated with down‐regulation of the FcεR1γ adaptor protein [one immunoreceptor tyrosine‐based activation motif (ITAM) each] and potential replacement with the CD3ζ adaptor protein (three ITAMS each). Additional changes not shown include epigenetic modifications (demethylation) activating the interferon‐γ locus and down‐regulation of Syk, Ewing's sarcoma's/FLI‐1 activated transcript (EAT) and move as indicated to end of sentence promyelocytic leukaemia zinc finger protein (PLZF) transcription factor. Although NK cells expressing NKG2C and CD57 have undergone differentiation and are considered adaptive NK in the setting of HCMV infection, specificity for HCMV has not been demonstrated. Both NKG2A and NKG2C interact with HLA‐E and CMV infection can increase expression of HLA‐E, but the role that either interaction plays in shaping the NK cell repertoire in CMV infection is unknown, hence a ‘?’ punctuates the dotted lines denoting receptor–ligand interactions.
