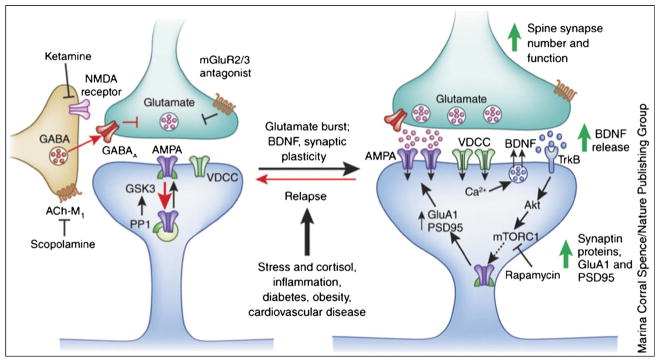
Fig. 5.
Ketamine causes a burst of glutamate that is thought to occur via disinhibition of GABA interneurons; the tonic firing of these GABA interneurons is driven by NMDA receptors, and the active, open-channel state allows ketamine to enter and block channel activity. The resulting glutamate burst stimulates AMPA receptors, which causes depolarization and activation of voltage-dependent Ca2+ channels (VDCC), leading to release of BDNF and stimulation of TrkB receptors and activation of Akt, which then increases mTORC1 signaling, leading to the increased synthesis of proteins that are required for synapse maturation and formation (i.e., GluA1 and PSD95). Under conditions in which BDNF release is blocked or neutralized, or in which mTORC1 signaling is blocked by rapamycin, the synaptic and behavioral actions of ketamine are blocked. Scopolamine also causes a glutamate burst via blockade of acetylcholine muscarinic M1 (ACh-M1) receptors on GABA interneurons. Antagonists ofmGluR2/3 also produce rapid antidepressant actions via blockade of presynaptic autoreceptors that inhibit the release of glutamate. Relapse to a depressive state is associated with a decrease of synapses on mPFC neurons, which could occur via stress and imbalance of endocrine hormones (cortisol), estrogen, inflammatory cytokines, and metabolic and cardiovascular illnesses (from [23•])