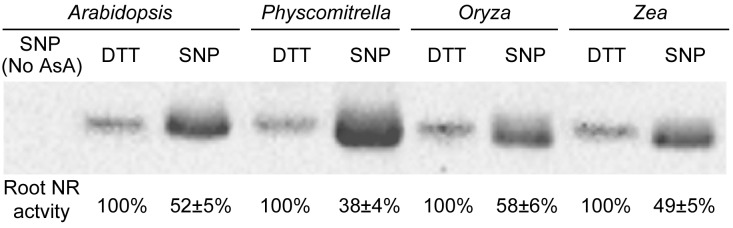
FIGURE 2.
S-Nitrosylation levels and root enzyme activities of nitrate reductase from four plant species. The 14-day-old Arabidopsis thaliana, Physcomitrella patens, Oryza sativa and Zea mays seedlings treated grown with or without 0.2 mM SNP (sodium nitroprusside; a NO donor) or 2 mM DTT (dithiothreitol; a NO inhibitor) were harvested for protein extraction. Total cellular proteins were extracted with HEN buffer (250 mM Hepes-NaOH pH 7.7, 1 mM EDTA, 0.1 mM neocuproine and proteinase inhibitor cocktail). After placing on ice for 20 min, the homogenate was centrifuged at 4°C at 14,000 rpm for 5 min. The supernatant was placed into a new tube and centrifuged it at 4°C at 14,000 rpm for 15 min. About 200 μg total cellular proteins were diluted into 125 μl HEN buffer with 500 μl blocking buffer [2.5% SDS and 20 mM methylmethane thiosulphonate (MMTS) in HEN buffer]. After 60 min incubation in 50°C under continuous shaking, MMTS was removed by acetone precipitation. The protein was diluted with 100 μl HEN buffer plus 1% SDS. The labeling reaction was initiated by adding 25 μl of 400 μM sodium ascorbate and 25 μl biotin-M (Sigma-Aldrich) and incubating at 25°C 2 h on a shaker. All proteins after biotin-switch were immunoprecipitated with the biotin antibody (Abcam), and then were subjected to Western blotting with the Arabidopsis NR antibody (Agrisera: AS08 310). 1/10 of the immunoprecipitated proteins (corresponding to 20 μg total cellular proteins) were loaded into each lane (Zhang et al., 2017). Biotin-switch assay and Western blotting were repeated 3 times, and typical results were presented. Protein extracts without ascorbate treatments were used as the negative control (No AsA). The NR activity was measured by mixing 1 volume of protein extracts with 5 volumes of pre-warmed (25°C) assay buffer (100 mM HEPES-KOH, pH 7.5, 5 mM KNO3, and 0.25 mM NADH). The reaction was started by the addition of assay buffer, incubated at 25°C for 30 min, and then stopped by adding 0.1 M zinc acetate. After 15 min, the tubes were centrifuged at 13,000 ×g for 5 min. The nitrite produced was measured at 520 nm by adding 1 mL of 1% (w/v) sulfanilamide in 3 M HCl plus 1 mL of 0.02% (v/v) N-(1-naphthyl)-ethylenediamine (Hu et al., 2015). Root NR activity of each plant treated with DTT was normalized to 100%. Enzyme activities under the SNP treatment are shown as mean ± standard deviations (n = 3).