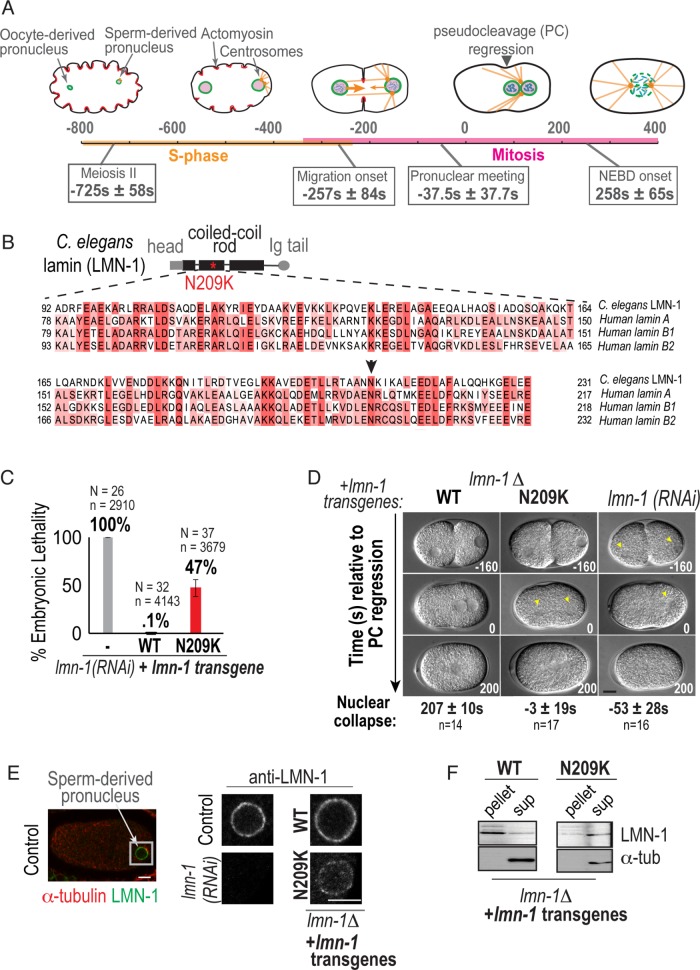
FIGURE 1:
A disease-causing mutation in C. elegans lamin (lamin-N209K) is hypomorphic for lamin function. (A) Schematic illustrating the timeline of pronuclear positioning events in the one-cell stage C. elegans embryo relative to pseudocleavage (PC) regression. Times of key events are the means of n = 8 embryos ± SD. (B) Schematic of C. elegans lamin (LMN-1) protein domain structure, relative position of N209K disease mutation (red asterisk), and multiple alignment of indicated coiled-coil domain in C. elegans and human lamins. Arrowhead marks conserved asparagine residue. (C) Percentage of embryonic lethality from the indicated conditions. N = number of worms, n = number of embryos. Data are represented as mean ± SD. (D) DIC images from time-lapse series of C. elegans one-cell embryos for the indicated conditions. Arrowheads mark cleared regions that correspond to nuclear contents. Scale bar, 10 μm. Times below are the mean times in seconds ± SEM of nuclear collapse from DIC images. Time points are in seconds relative to PC regression. (E) Representative example of immunofluorescence image of a wild-type one-cell embryo stained for lamin (LMN-1) (red) and α-tubulin (green). Magnified image of sperm-derived pronucleus immunostained with antibody against endogenous LMN-1 from indicated conditions. Scale bars, 5 μm. (F) Immunoblot probed for LMN-1 and α-tubulin after preparation of lysates into soluble (sup) and insoluble (pellet) fractions of worms from indicated genetic backgrounds (N = 3). See also Supplemental Figure S1.