Abstract
Daily exposure to low doses of 3-methylcholanthrene (3MC) during the pubertal period in rats disrupts both follicular growth and ovulation. Thus, to provide new insights into the toxicity mechanism of 3MC in the ovary, here we investigated the effect of daily exposure to 3MC on selected ovarian genes, the role of the aryl hydrocarbon receptor (AhR) and the level of epigenetic remodeling of histone post-transcriptional modifications. Immature rats were daily injected with 3MC (0.1 or 1 mg/kg) and mRNA expression of genes involved in different ovarian processes were evaluated. Of the 29 genes studied, 18 were up-regulated, five were down-regulated and six were not altered. To assess whether AhR was involved in these changes, we used the chromatin immunoprecipitation assay. 3MC increased AhR binding to promoter regions of genes involved in Notch signaling (Hes1, Jag1), activation of primordial follicles (Cdk2), cell adhesion (Icam1), stress and tumor progression (Dnajb6), apoptosis (Bax, Caspase-9) and expression of growth and transcription factors (Igf2, Sp1). By studying the trimethylation and acetylation of histone 3 (H3K4me3 and H3K9Ac, respectively) of these genes, we found that 3MC increased H3K4me3 in Cyp1a1, Jag1, Dnajb6, Igf2, Notch2, Adamts1, Bax and Caspase-9, and H3K9Ac in Cyp1a1, Jag1, Cdk2, Dnajb6, Igf2, Icam1, and Sp1. Co-treatment with α-naphthoflavone (αNF), a specific antagonist of AhR, prevented almost every 3MC-induced changes. Despite the low dose used in these experiments, daily exposure to 3MC induced changes in both gene expression and epigenomic remodeling, which may lead to premature ovarian failure.
Keywords: 3-methylcholanthrene, α-naphthoflavone, aryl hydrocarbon, ovary, gene expression
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are organic chemical compounds containing carbon and hydrogen, arranged in aromatic rings, and found in coal and tar deposits. They are released into the environment by different natural or anthropogenic activities, particularly from incomplete combustion in the incineration of organic matter like wood, oil, tobacco, foods, or vehicular emissions. Although PAHs may be numerous and may vary in the number and arrangement of rings, the most known are derived from anthracene, phenanthrene and pyrene. PAHs are considered important environmental pollutants and many of them are of interest due their mutagenic, carcinogenic and/or teratogenic properties. In addition, at low doses, some of these PAHs have a direct action on the reproductive function of female mammals. It is well known that three of them, benzo[a]pyrene (BaP), 9,10-dimethylbenz[a]anthracene (DMBA) and 3-methylcholanthrene (3MC), are potent ovotoxic substances since they destroy immature follicles in mice and rats (Mattison 1980; Shiromizu and Mattison 1985; Borman et al. 2000) and cause early ovarian failure. Moreover, exposure to these PAHs has been linked to premature menopause in women who smoke (Mattison and Thorgeirsson 1979; Shiromizu and Mattison 1985; Freour et al. 2008; Sun et al. 2012).
Most PAHs with coplanar structure exert their toxic action mediated by the aryl hydrocarbon receptor (AhR), although some of them may also act through different pathways (Fujii-Kuriyama and Mimura 2005). AhR is a ligand-activated transcription factor and the first protein involved in a signaling cascade of events that induce the expression of several genes involved in different processes, including embryonic development, cell proliferation and differentiation, and of genes coding for xenobiotic-metabolizing enzymes such as those belonging to the cytochrome P450 (Cyp) family (Hahn 2002; Shimada 2006). AhR is present in different ovarian cells such as follicles, oocytes, and granulosa and theca cells, in multiple species, including humans (Chaffin et al. 1996; Robles et al. 2000; Davis et al. 2000; Khorram et al. 2002; Hasan and Fischer 2003; Baba et al. 2005; Baldridge and Hutz 2007; Jablonska et al. 2011)
Some reproductive processes such as fertility, gametogenesis, follicular development (Benedict et al. 2000, 2003; Barnett et al. 2007a, 2007b), and ovarian function (Hernández-Ochoa et al. 2009, 2010) are dependent on the activation of AhR. Previously, we found that daily exposure to low doses of 3MC during the pubertal period in rats disrupt both follicular growth and ovulation through an AhR-dependent mechanism (Rhon-Calderón et al. 2016). Therefore, and to provide new insights into the toxicity mechanism of 3MC in the ovary, in the present study, we investigated the effect of daily low doses of 3MC on the expression of selected groups of ovary-expressed genes enriched in putative AhR binding sites, in rats. We also determined the effect of 3MC on AhR binding to 5′ regulatory regions of selected genes in the ovary, as well as the level of histone modifications (H3K4me3 and H3K9Ac) as a readout of promoter activation. Finally, we determined the ability of α-naphthoflavone (αNF) to prevent the genomic actions of 3MC in the ovarian tissue.
MATERIAL AND METHODS
Drugs and chemicals
3MC (purity: 99.8%), αNF (> 98% purity), and other biochemical reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA). Primers (Supplementary table 1) were purchased from Eurofins MWG Operon (Huntsville, AL, USA).
Animals
Immature female Sprague–Dawley rats aged 20 days were purchased from the School of Veterinarian Sciences of Buenos Aires University, Argentina. Animals were maintained under controlled conditions of light (12 h light/12 h darkness), temperature (22 °C) and humidity, with free access to food and water. All animals were handled according to the Guiding Principles for the Care and Use of Research Animals, and all protocols were approved by the Institutional Committee of the School of Medicine of Buenos Aires University, Argentina (CICUAL) by Resolution 1928/14.
Experimental design
At 21 days of age, female rats were weighed, randomly distributed into different experimental groups, and daily intraperitoneally injected with 3MC (0.1 or 1 mg/kg) and/or αNF (80 mg/kg). Corn oil vehicle (2.0 ml/kg) was used as vehicle. Administration of 3MC and vehicle started at 22 days of age, whereas, to insure αNF action, administration of αNF started at 21 days of age. All treatments were completed at 40 days of age. All animals (six to eight per group) were killed by decapitation in the afternoon of the second estrous cycle. Doses were selected based on previous studies showing that they are effective to destroy primordial follicles and inhibit both follicular growth and ovulation (Borman et al. 2000; Rhon Calderón et al. 2016).
RNA isolation and real time-Polymerase chain reaction (real time-PCR)
Total RNA was isolated from frozen ovaries by using Quick-RNATM MiniPrep (Zymo Research, Irvine, CA, USA), according to the manufacturer’s instructions. RNA samples were obtained as described previously (Rhon-Calderón et al. 2016). Briefly, RNA (1000 ng) from the rat ovary was reverse transcribed (RT) using the Omni RT Kit (Qiagen, Valencia, CA, USA) in the presence of random hexamer primers, as recommended by the manufacturer (Invitrogen, Carlsbad, CA, USA). All real-time PCR reactions were performed using a QuanStudio 12 K Real-Time PCR system. To determine the relative abundance of the mRNAs of interest, we used the SYBR GreenER qPCR SuperMix system (Invitrogen). Primers for amplification were designed using the PrimerSelect tool of DNASTAR 11 software (Madison, WI, USA) or the NCBI online Primer-Blast program (Supplementary table 1). PCR reactions were performed in a total volume of 10 μl, each reaction containing 1 μl of diluted cDNA or a reference cDNA sample, 5 μl of SYBR GreenER qPCR SuperMix and 4 μl of a primer mix (300 nM of each gene specific primer). The PCR conditions used were 95 °C for 5 min, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C. To confirm the formation of a single SYBR Green-labeled PCR amplicon, the PCR reaction was followed by a three-step melting curve analysis consisting of 15 s at 95 °C, 60 s at 60 °C, ramping up to 95 °C at 0.5 °C.s−1, detection every 0.5 s and finishing 15 s at 95 °C, as recommended by the manufacturer. Experiments were performed three times. Data were normalized to ribosomal 18s mRNA in each sample.
Chromatin immunoprecipitation (ChIP) assay
To assess the recruitment of AhR to different gene promoters, we performed ChIP assays by using chromatin extracted from the ovarian tissue, as described previously (Lomniczi et al. 2013; Rhon-Calderón et al. 2016). Briefly, ovarian tissue (20 mg) was cut into small fragments in ice-cold PBS containing a protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 7 μg.ml−1 aprotinin, 0.7 μg.ml−1 pepstatin A, 0.5 μg.ml−1 leupeptin), a phosphatase inhibitor cocktail (1 mM β-glycerophosphate, 1 mM sodium pyrophosphate and 1 mM sodium fluoride), and a histone deacetylase (HDAC) inhibitor (20 mM sodium butyrate). Cross-linking was performed by incubating the tissue fragments in 1% formaldehyde for 10 min at room temperature. Tissue fragments were lysed with 200 ml SDS buffer (0.5% SDS, 50 mM Tris–HCl, 10 mM EDTA) containing protease, phosphatase and HDAC inhibitors, and sonicated for 40 s to yield chromatin fragments of ≅ 500 bp. Size fragmentation was confirmed by agarose gel electrophoresis. Aliquots of chromatin were incubated with 5 μg antibody (Supplementary table 2). Antibody–chromatin complexes and 25 ml of protein A or G beads solution (Dynabeads) were incubated at 4 °C overnight with gentle agitation. DNA was recovered by using the ChIP DNA Clean and Concentrator columns (Zymo Research) and stored at −80 °C until subsequent PCR analysis. Genomic regions of interest were amplified by qPCR. Supplementary table 1 shows the primer sequences (Eurofins MWG Operon, Huntsville, AL, USA) used to detect the DNA fragment of interest in the immunoprecipitated DNA. For semiquantitative detection, PCR reactions were performed using SYBR Green ER (Invitrogen) with each immunoprecipitated DNA or input samples. The thermocycling conditions used were 95 °C for 5 min, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C. Data are expressed as percent recruitment relative to chromatin input. Experiments were performed three times.
Statistical analysis
All data are expressed as means ± S.E.M. Statistical analysis was performed using two-way ANOVA with Bonferroni post-test. Levene’s test and a modified Shapiro–Wilk test were used to assess homogeneity of variances and normal distribution, respectively. Differences between groups were considered significant when P<0.05. Statistical graphs were generated using Graph Pad Prism 5.0 (La Jolla, CA, USA).
RESULTS
mRNA expression of different genes involved in the ovarian function
Considering that many PAHs act through AhR and that daily in vivo exposure to 3MC during the pubertal period induces the ovarian expression of metabolizing enzymes, such as Cyp1a1 and Cyp1b1, as demonstrated previously (Rhon-Calderón et al. 2016), here we studied the effects of 3MC and αNF on the expression of several genes involved in the ovarian function. To look for potential target genes, we used the match algorithm of Transfac 7.0 to mine for AhR sites (5′-T/GNGCGTGA/CG/CA-3′) present between position −1000 and position +500 of the TSS (Dere et al. 2011) of all 4364 genes involved in the ovarian function according to the Ovarian Kaleidoscope database. A total of 825 genes were found to contain potential AhR sites (Supplementary table 3). Functional cluster analysis and Kegg pathway analysis using David 6.8 showed enrichment in apoptosis-related genes, protein kinases, transforming growth factor signaling molecules, nuclear and steroid hormone receptors, epidermal growth factor signaling molecules and adhesion molecules (Supplementary table 4).
First, we evaluated the mRNA expression of genes involved in steroidogenesis and steroid receptor signaling (Esr1), notch signaling (Hes1, Jag1, Dll1 and Notch2), activation of primordial follicles (Cdk2, Akt1), cell adhesion (Icam1, Itga4, Itgb8 and Adamts1), cell cycle and differentiation (Foxn3, Ddx5, Gdf9), oocyte-spermatozoa interaction (Cd9), stress, tumor progression and metastasis (Dnajb6), apoptosis (Bax, Caspase-9), expression of receptors and other kinases (Egfr, Fgfr1, Igf2, Mapk1), and expression of other cell signaling molecules (Bmp15, Bmp4, Foxo3, Ddr2, Sp1, Calr and Hspa8), most of which contained predicted AhR consensus sequences in the promoter regions (Table 1). Exposure to both doses of 3MC significantly altered the expression of most of the genes studied compared with controls (Table 1). Of the 29 genes studied, 18 were up-regulated, five were down-regulated and six were not altered. αNF treatment clearly prevented almost all of these alterations, except in Egfr, Bmp4 and Caspase-9 at the higher dose of 3MC.
Table 1.
Effect of daily treatments with 3-methylcholanthrene (3MC, 0.1 and 1 mg/kg) and/or α-naphthoflavone (αNF, 80 mg/kg), or vehicle (Control) on the mRNA from genes involved in the ovarian function. 3MC and vehicle treatments started at 22 days of age whereas αNF started at 21 days of age. All treatments were completed at 40 days of age. Data were normalized to ribosomal 18s mRNA in each sample and represent the mean ± S.E.M. for 6-8 ovaries from different animals with the same treatment.
| Genes | Control | αNF | 3MC (0.1) | 3MC (0.1) + αNF | 3MC (1) | 3MC (1) + αNF |
|---|---|---|---|---|---|---|
| Hes1 | 0.88±0.06 | 0.80±0.09 | 1.3±0.1*** | 0.91±0.04## | 1.5±0.1*** | 0.97±0.06### |
| Jag1 | 0.97±0.07 | 0.98±0.05 | 1.25±0.07** | 0.83±0.06### | 1.29±0.05*** | 0.82±0.05### |
| Dll1 | 0.9±0.1 | 0.8±0.1 | 2.8±0.1*** | 0.9±0.1### | 3.03±0.07*** | 0.9±0.1### |
| Notch2 | 0.9±0.11 | 0.8±0.1 | 1.6±0.1*** | 0.90±0.09### | 1.7±0.1*** | 0.9±0.1### |
| Mapk1 | 1.0±0.2 | 0.9±0.2 | 0.8±0.1 | 0.8±0.1 | 0.7±0.1 | 0.65±0.07 |
| Cdk2 | 0.96±0.08 | 1.0±0.1 | 1.44±0.06** | 1.0±0.1## | 1.6±0.2*** | 0.9±0.1### |
| Dnajb6 | 0.87±0.09 | 0.9±0.1 | 1.7±0.1*** | 0.76±0.09### | 1.5±0.1*** | 0.91±0.09### |
| Akt1 | 0.96±0.08 | 1.0±0.1 | 1.53±0.07*** | 1.0±0.1## | 1.6±0.1*** | 0.9±0.1### |
| Egfr | 1.0±0.2 | 1.1±0.1 | 0.8±0.1 | 0.75±0.08 | 0.5±0.1* | 0.50±0.07# |
| Igf2 | 0.87±0.07 | 0.9±0.1 | 1.6±0.2*** | 0.8±0.1### | 1.5±0.1** | 0.9±0.1## |
| Icam1 | 0.89±0.08 | 0.8±0.1 | 1.6±0.1*** | 0.88±0.09### | 1.4±0.1*** | 0.85±0.06### |
| Carl | 0.87±0.06 | 0.81±0.08 | 1,4±0.1*** | 0.85±0.09### | 1.4±0.1** | 0.73±0.05### |
| Hspa8 | 0.87±0.09 | 0.9±0.1 | 1.4±0.1** | 0.76±0.09### | 1.35±0.07** | 0.91±0.09# |
| Ddr2 | 0.76±0.08 | 0.7±0.1 | 1.5±0.2** | 0.9±0.2## | 1.49±0.08** | 1.0±0.1## |
| Fgfr1 | 1.00±0.23 | 0.85±0.15 | 0.76±0.13 | 0.65±0.03 | 0.59±0.07 | 0.54±0.07 |
| Sp1 | 0.96±0.04 | 0.95±0.06 | 1.21±0.05** | 0.91±0.05### | 1.23±0.04*** | 0.99±0.06## |
| Bmp4 | 1.0±0.2 | 0.92±0.09 | 1.0±0.1 | 0.9±0.1 | 0.59±0.06* | 0.8±0.1 |
| Itga4 | 0.88±0.09 | 0.9±0.1 | 0.80±0.09 | 0.81±0.09 | 0.73±0.04 | 0.75±0.06 |
| Ddx5 | 0.89±0.07 | 0.8±0.1 | 1.7±0.2*** | 0.84±0.08### | 1.69±0.09*** | 0.97±0.08### |
| Foxn3 | 0.9±0.1 | 0.89±0.07 | 1.35±0.09*** | 0.84±0.06### | 1.41±0.07*** | 0.93±0.04### |
| Esr1 | 0.88±0.06 | 0.85±0.07 | 0.51±0.05*** | 0.81±0.05## | 0.54±0.02*** | 0.82±0.06## |
| Itgb8 | 0.89±0.09 | 0.85±0.09 | 0.44±0.07*** | 0.79±0.05## | 0.39±0.02*** | 0.79±0.09## |
| Cd9 | 1.0±0.1 | 0.97±0.05 | 0.50±0.05*** | 1.0±0.1### | 0.5±0.1*** | 1.0±0.1### |
| Adamts1 | 0.97±0.09 | 0.87±0.05 | 1.80±0.02*** | 0.87±0.02### | 1.79±0.03*** | 0.84±0.04### |
| Gdf9 | 0.88±0.06 | 1.6±0.1*** | 0.91±0.06 | 1.5±0.1*** | 0.81±0.06 | 1.5±0.1*** |
| Bmp15 | 0.88±0.06 | 1.3±0.1** | 0.89±0.06 | 1.4±0.1*** | 0.82±0.07 | 1.38±0.04*** |
| Foxo3 | 0.88±0.07 | 1.25±0.04** | 0.94±0.08 | 1.42±0.07*** | 0.86±0.09 | 1.34±0.07*** |
| Bax | 1.00±0.05 | 1.5±0.2 | 37±4*** | 5.7±0.7### | 3.8±0.5 | 2.5±0.2 |
| Caspase9 | 1.0±0.2 | 0.7±0.2 | 4.4±0.5*** | 1.4±0.4## | 3.3±0.8** | 3.0±0.6** |
P<0.01 and
P<0.001 versus Control,
P<0.05,
P<0.01 and
P<0.001 versus corresponding 3MC (two-way ANOVA with Bonferroni post-test)
AhR binding to upstream regulatory regions of ovary-expressed genes
To study whether 3MC- and/or αNF-induced changes in mRNA expression were mediated by recruitment of AhR to upstream regulatory regions of ovary-expressed genes, we then performed ChIP-PCR assays for AhR, as described previously (Rhon-Calderón et al. 2016). The daily treatment of 3MC increased AhR binding to promoter regions of most of the genes studied, except for Notch2, Akt1 and Adamts1 (data not shown). Treatment with αNF prevented the 3MC-induced increase in AhR binding, while no changes were found in rats that received αNF alone (Fig. 1).
Fig. 1.
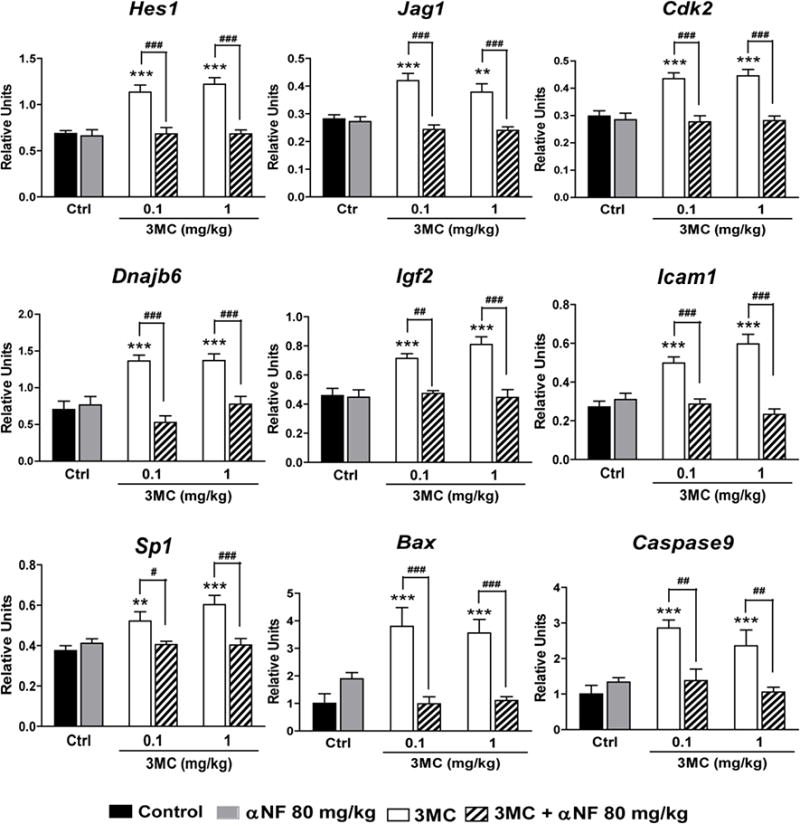
Effect of daily treatments with 3-methylcholanthrene (3MC) and/or α-naphthoflavone (αNF), or vehicle (Ctrl) on the recruitment of AhR to promoter region of genes involved in the ovarian function, as determined by the chromatin immunoprecipitation assay. Results are expressed as percent pull down relative to input chromatin. 3MC and vehicle treatments started at 22 days of age, whereas αNF treatment started at 21 days of age. All treatments were completed at 40 days of age. Data represent the mean ± S.E.M. for 6-8 ovaries from different animals with the same treatment. **P<0.01 and ***P<0.001 versus Ctrl, ##P<0.01 and ###P<0.001 versus the corresponding 3MC concentrations (two-way ANOVA with Bonferroni post-test)
Post-translational modifications in upstream regulatory regions of ovary-expressed genes
To explore whether AhR-induced activation of gene expression was accompanied by epigenetic remodeling of histone post-transcriptional modifications (PTMs), we studied two PTMs apparently linked to gene activation: trimethylation of histone 3 at lysine 4 (H3K4me3), a PTM associated with the trithorax group of transcriptional activators (Fig. 2), and acetylation of histone 3 at lysine 9 (H3K9Ac), a PTM induced by histone acetyl transferases (Fig. 3), both of which have been previously found to be associated with remodeling of the Cyp1a1 and Cyp1b1 promoters (Schnekenburger et al. 2007; Beedanagari et al. 2010). Since we have previously found that 3MC treatment causes an AhR-mediated induction of the mRNA expression of two important metabolizing enzymes, Cyp1a1 and Cyp1b1, in ovarian tissue (Rhon-Calderón et al. 2016), these were included in this part of the study. The daily treatment with 3MC increased H3K4me3 in Cyp1a1, Jag1, Dnajb6, Igf2, Notch2, Adamts1, Bax and Caspase-9, decreased H3K4me3 in Cdk2, and did not change H3K4me3 in Cyp1b1 or Akt1 (data not shown), as compared to controls. We did not detect H3K4me3 in the promoter region of Hes1, Icam1 or Sp1 (data not shown). In turn, 3MC treatment increased H3K9Ac in Cyp1a1, Jag1, Cdk2, Dnajb6, Igf2, Icam1, Sp1, decreased H3K9Ac in Notch2, Akt1 and Adamts1 and did not change H3K9Ac in Cyp1b1, Itga4, Bax and Caspase-9 (data not shown) as compared to controls. We did not observe H3K9Ac in the promoter of Hes1 (data not shown). αNF treatment prevented the 3MC-induced changes in histone PTMs, except H3K9Ac in the Notch2 promoter. Moreover, αNF treatment alone also induced changes in trimethylation and acetylation of some promoter regions (Figs 2 and 3).
Fig. 2.
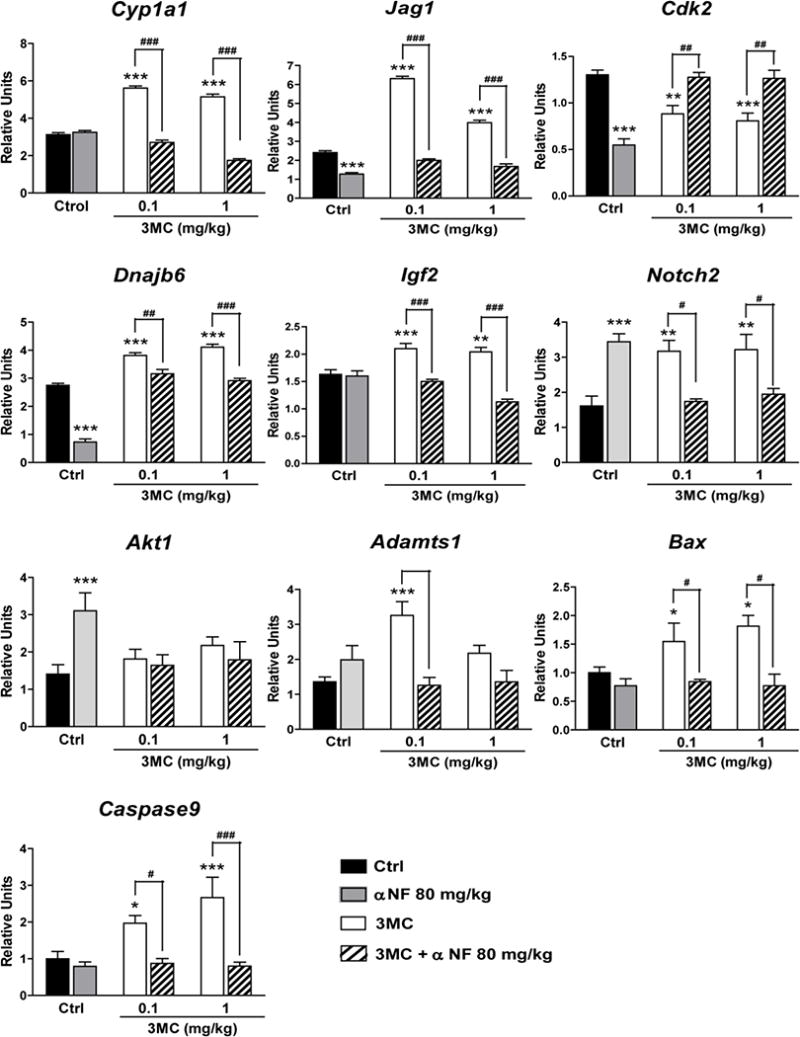
Effect of daily treatments with 3-methylcholanthrene (3MC) and/or α-naphthoflavone (αNF), or vehicle (Ctrl) on the trimethylation of histone 3 at lysine 4 on the promoter region of genes involved in the ovarian function, as determined by the chromatin immunoprecipitation assay. Results are expressed as percent pull down relative to input chromatin. 3MC and vehicle treatments started at 22 days of age, whereas αNF treatment started at 21 days of age. All treatments were completed at 40 days of age. Data represent the mean ± S.E.M. for 6 ovaries from different animals with the same treatment. **P<0.01 and ***P<0.001 versus Ctrl, ##P<0.01 and ###P<0.001 versus the corresponding 3MC concentrations (two-way ANOVA with Bonferroni post-test)
Fig. 3.
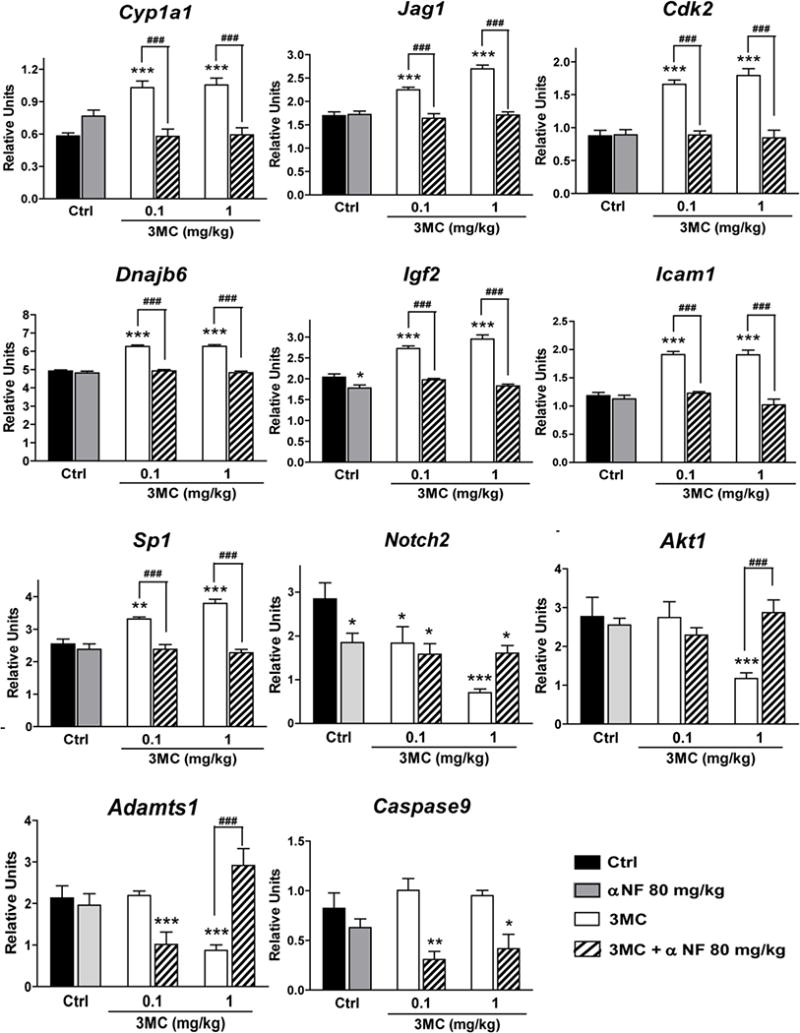
Effect of daily treatments with 3-methylcholanthrene (3MC) and/or α-naphthoflavone (αNF), or vehicle (Ctrl) on the acetylation of histone 3 at lysine 9 on the promoter region of genes involved in the ovarian function by the chromatin immunoprecipitation assay. Results are expressed as percent pull down relative to input chromatin. 3MC and vehicle treatments started at 22 days of age, whereas αNF treatment started at 21 days of age. All treatments were completed at 40 days of age. Data represent the mean ± S.E.M. for 6 ovaries from different animals with the same treatment. **P<0.01 and ***P<0.001 versus Ctrl, ###P<0.001 versus the corresponding 3MC concentrations (two-way ANOVA with Bonferroni post-test)
DISCUSSION
Previously, we found that daily exposure to 3MC during the pubertal period reduces the follicle reserve, follicle growth and ovulation, and that co-exposure to αNF prevents the gonadal 3MC-induced toxicity (Rhon-Calderón et al. 2016). Thus, in the present study, we tested the hypothesis that daily treatment with 3MC leads to alterations in the expression of genes involved in the formation of the follicle reserve, follicular development and/or ovulation as well as apoptosis. We also tested the hypothesis that the 3MC-induced actions are mediated through recruitment of AhR to 5′ regulatory regions of ovary-expressed genes and that this process involves epigenetic rearrangement that favors an open chromatin state.
Consistent with previous studies, we observed that 3MC-induced ovarian toxicity modified the expression of genes involved in folliculogenesis, cell cycle, cell proliferation, survival/apoptosis, stress and cell communication. Similarly, using neonatal mice daily exposed to 3MC (and other PAHs, which also target immature follicles), or 5-week-old mice exposed to cigarette smoke, Sobinoff et al. (2011, 2012a, 2012b, 2013) showed changes in the ovarian expression of genes involved in different cellular processes such as biotransformation, cell cycle regulation, cell death, cell to cell signaling, cell growth and proliferation, cell cycle progression, free radical scavenging, drug metabolism, and genetic and reproductive disorder. These results indicate that 3MC exposure not only destroys ovarian follicles but also interferes in many physiological processes of the ovarian function. To provide insights into the mechanisms by which 3MC induces ovotoxicity, we studied whether 3MC regulates these genes by activation of AhR. To this end, we limited this study to genes closely related to AhR transcriptional activity and involved in the ovarian function.
Overall, PAH-induced ovotoxicity, particularly oocytes loss and follicular growth, is considered to be mediated by AhR activation. This statement is supported by the fact that PAH exposure increases the expression of AhR protein level in ovarian cells (Jung et al. 2010) and the fact that the presence or administration of αNF prevents PAH-induced ovotoxicity (Mattison and Thorgeirsson 1979; Shiromizu and Mattison 1985; Thompson et al. 2005; Rhon-Calderón et al. 2016). These results, together with those showing that AhR-deficient mice exhibit an increased number of germ cells and primordial follicles, a decreased number of antral follicles (Benedict et al. 2000) and corpora lutea (Benedict et al. 2003), and slower follicle growth (Barnett et al. 2007b), seem to indicate that i) AhR signaling is essential in the formation of germ cells and follicle development, and that ii) in the ovary, the action of PAHs is mainly mediated through AhR signaling. However, some PAH actions may be mediated by an AhR-independent mechanism. Sadeu et al. (2013) found that although the expression of both Cyp1a1 and Cyp1b1 mRNA was increased, there were no changes in the expression of AhR mRNA in ovarian follicles from mice exposed to BaP at any stage of development. In a recent work, and after verifying that 3MC induced the mRNA expression of Cyp1a1 and Cyp1b1, we used the ChIP assay to detect the binding of AhR to the promoter region of both genes (Rhon-Calderón et al. 2016). In that study, we demonstrated, for the first time, the direct participation of AhR in inducing both enzymes in ovarian tissue after daily exposure to 3MC. In the present work, we studied whether the genomic changes found after daily 3MC exposure in the ovarian tissue were directly associated with AhR activation.
Cyclin-dependent kinases (CDKs) are a family of protein kinases involved in regulating the cell cycle. To be active, CDKs have to bind to regulatory proteins, known as cyclins, which function as serine-threonine kinases (Morgan 1997). Of special interest, CDK2 is known to be activated as CDK2-cyclin E at the beginning of the S phase to induce the initiation of DNA synthesis, and then binds cyclin A throughout S phase progression (Morgan 1997). This protein is associated with both the formation and activation of primordial follicles and the follicle survival rate (Rajareddy et al. 2007). Thus, the up-regulation of the gene coding for this protein seems to contrast with our previous results where rats exposed to 3MC exhibit a lower number of primordial, preantral and antral follicles in parallel with lower ovulation rate, compared with controls (Rhon-Calderón et al. 2016). However, using specific markers of follicular development and atresia, Sobinoff et al. (2011, 2012a, 2012b, 2013) reported that both in vitro and in vivo exposure to PAHs, including 3MC, induces excessive activation and loss of primordial follicles and development of follicular atresia. These results indicate that the increased expression of Cdk2, which is involved in activating primordial follicles and cell proliferation, may be a compensatory mechanism to maintain a constant flow of developing follicles (Sobinoff et al. 2012a, 2012b).
It is no surprise that 3MC exposure induces the expression of Dnaj and Igf2. Accumulated evidences have shown that 3MC is a potent carcinogen through the action of its oxidized metabolites (Sims 1966, 1970; Cavalieri et al. 1978; Flesher et al. 1998; Shimada 2006), some of which are generated by CYP enzymes. Moreover, we have previously found that both doses of 3MC induce the formation of micronuclei and degradation of DNA in both oocytes and bone marrow cells in rats (Rhon Calderón et al. 2016). Dnaj (an Hsp40 homolog), subfamily B, member 6, also known as Mrj, is ubiquitously expressed and encodes a member of co-chaperone proteins that function with Hsp70 proteins to protect proteins from aggregation during protein synthesis and cellular stress (Fan et al. 2003; Meng et al. 2016). Murine Dnajb6 is expressed in the placenta, several regions of the embryo, and some tissues of adults. It is essential for the formation of the chorioallantoic placenta (Hunter et al. 1999). However, recent evidence has shown that Dnajb6 is involved in different aspects of tumor progression and metastasis. In invasive ductal carcinoma, DNAJB6 (Mrj) is lost and its restoration restricts the malignant behavior of breast cancer and melanoma cells (Mitra et al. 2008). However, overexpression of Dnajb6 promotes colorectal cancer cell invasion, whereas its silencing inhibits the invasion of these malignant cells (Zhang et al. 2015). IGF2 is a growth factor involved in the development and proliferation of human cancer cells (Livingstone 2013; Brouwer-Visser and Huang 2015). Increased serum IGF2 levels are associated with an increased risk of developing various cancers, and it has been shown that IGF-2 up-regulates AhR mRNA and protein levels, which, in turn induce the expression of Cyclin D1, increasing cell proliferation. Thus, it is likely that the increased expression of both Dnajb6 and Igf2 genes, observed in the present study, is associated with a future tumor progression induced by 3MC exposure.
Notch signaling is involved in many cellular processes, including cell-fate specification, cell-cell communication, follicle assembly and growth, survival and apoptosis (Vanorny and Mayo 2017). The inhibition or disruption of the Notch signaling is involved in different carcinogenic processes in several tissues, including the ovarian tissue (Groeneweg et al. 2014; Xie et al. 2017). Recent studies have shown that Notch knockout mice exhibit loss of primordial follicles, reduction in both growing and antral follicles, and a low production of corpora lutea (Vanorny et al. 2014). Likewise, a decrease in granulosa cell proliferation and an increase in cell apoptosis have been observed in the ovaries of both Jag1 and Notch2 knockout mice. In a previous study, we found that rats exposed daily to 3MC exhibited a follicular composition similar to that of these knockout mice, as these animals had a low number of primordial follicles, growing and antral follicles, and a lower ovulation rate than control animals (Rhon-Calderón et al. 2016). It was surprising that, in the present study, we observed an increase in the mRNA expression of all the mediators of Notch signaling measured (Hes1, Jag1, Dll1 and Notch2). However, it is possible that these increases are compensatory changes induced by the disruption of the Notch signaling pathway. This possibility is consistent with the findings of Vanorny et al. (2014), who showed that Notch2 mRNA levels are increased in Jag1 knockout ovaries and that, conversely, the levels of Jag1 are increased in Notch2 knockout ovaries. These authors postulated that these effects are part of a compensatory mechanism or a functional complementation between Notch2 and Jag1.
A crosstalk between Notch signaling and AhR activation has been recently described. Thomsen et al. (2004) found that Hes1 is up-regulated by 2,3,7,8-tetrachlorodibenzo-p-dioxin, a specific and potent AhR ligand, at both protein and mRNA levels in human mammary carcinoma cells. Xia et al. (2015) demonstrated, by both in vitro and in vivo studies, that traffic-related ultrafine particles, enriched in PAHs, induce Jag1 expression through a mechanism involving direct transcriptional activation of the Jag1 promoter. Xia et al. (2015) also found that treatment with a selective AhR inhibitor or deletion of the AhR gene abrogates particulate matter-induced Jag1 expression. Alam et al. (2010) identified a Notch–AhR axis that regulates IL-22 expression and fine-tunes the immune system control of inflammatory responses. Huang et al. (2016) found that the expression of Notch receptors and their target Hes1, but not Jag1, are markedly reduced in AhR−/− mouse testis, which exhibits low fertility with degenerative changes, germ cell apoptosis, and reduction of the number of spermatids and testosterone levels. In our study, we found that the increased expression of the Notch target gene Hes1 was AhR-dependent as determined by the ChIP assay. This result is consistent with that of Thomsen et al. (2004) who observed that Hes1 and the AhR complex compete for binding to the AhR response element in the upstream regulatory region of human Hes1. Further, the increased expression of the Notch ligand Jag1 is also AhR-dependent and, as indicated previously, it is possible that AhR regulates the expression of Jag1 in a Notch-independent way (Huang et al. 2016). Taken together, these data show, for the first time, the existence of a crosstalk between AhR and Notch signaling involved in follicular growth and ovulation in the rat ovary.
Cytokines are produced by different cell types, including immune cells, which are involved in different ovarian events such as folliculogenesis, ovulation and luteolysis. Lymphocytes and macrophages interact with ovarian cells, at least in part, through adhesion molecules such as ICAM1 (Viganò et al. 1997). ICAM1 is a cytokine mainly present in leukocytes and endothelial cells with a significant role as adhesion molecule involved in inflammatory processes. ICAM1 is expressed in ovarian cells, including oocytes (Olson et al. 2000), theca (Bonello et al. 2004) and granulosa cells, and follicular fluid (Viganò et al. 1997, 1998). Our results are in agreement with those of Oesterling et al. (2008), who found that BaP increases the protein expression of ICAM1 and induces the monocyte adhesion in human endothelial cells through an AhR- and caveolae-dependent mechanism. Furthermore, in vivo treatment with αNF (Oesterling et al. 2008) or the presence of other flavonoids (Owens et al. 2009), in in vitro experiments, block the induction of ICAM1 by BaP, confirming the involvement of AhR in this process. Considering that 3MC exposure leads to the destruction of primordial follicles and inhibition of folliculogenesis and ovulation, the increase in Icam1 gene expression may be part of the mechanism by which 3MC exposure would induce a toxic environment, likely due to the presence of oxidizing species, which would trigger cell death by apoptosis. This is consistent with the increase observed in the expression of both Bax and caspase-9 mRNA, which in turn, is supported by Matikainen et al. (2001, 2002), who demonstrated that exposure of mice to PAHs induces the expression of BAX in oocytes, followed by apoptosis and that this ovarian damage is prevented by AhR inactivation with αNF. Others have demonstrated that different PAHs induce the expression of pro-apoptotic proteins or their transcripts. Treatment of Hepa1c1c7 cells with BaP causes an increase in cell apoptosis, apparently via an increase in the enzymatic activation of caspase-3 and caspase-9, BAX expression, and mitochondrial function (Ko et al. 2004). The presence of PAHs increases the levels of both mRNA and protein of BAX in cultured rat ovaries (Ganesan and Keating 2014) and follicles (Sadeu et al. 2013), respectively.
Sp1 is a regulatory factor, and binding of Sp1 to the promoter region is essential for the regulation of AhR transcription (Kobayashi et al. 1996; Tang et al. 2010). After binding of the AhR-Arnt complex to the promoter element of the target genes, known as xenobiotic responsive element (XRE), Sp1 binds the basic transcription element (BTE) regulatory sequence to induce the transcription activity. XRE and BTE together are necessary to induce the expression of the gene targets in response to AhR activation by different PAHs. Kobayashi et al. (1996) demonstrated that either the binding of the AhR-Arnt heterodimer or Sp1 to their DNA sequences induces DNA binding of its cognate DNA element. Thus, these two transcription factors act in a synergistic transactivation manner. Our results are consistent with these data since we observed that Sp1 expression was increased by both doses of 3MC.
In the present study, we observed that exposure to 3MC also altered the epigenomic landscape preferentially at promoter regions of genes related to biotransformation of xenobiotics (Cypa1a), Notch signaling (Jag1), stress and tumor progression (Dnajb), apoptosis (Bax and Caspase-9), cell survival (Cdk2), and expression of growth factors (Igf2), transcription factor (Sp1) and adhesion molecules (Adamts1, Icam1). Acetylation of histones H3 (H3K9Ac) and trimethylation of H3 lysine4 (H3K4me3) are associated with active transcription. However, the association and interdependence between both post-transcriptional modifications is unknown. Ha et al. (2011) found that a histone H3 methyltransferase controls DNA methylation, whereas Lawrence et al. (2004) found that histone acetylation affects histone methylation. In a recent review, and after an exhaustive analysis of the studies so far, Howe et al. (2017) noted that H3K4me3 can be associated with both activation and repression of transcription at a small subset of genes, since H3K4me3 can recruit different factors whose interactions may lead to the activation or repression of transcription at different genes. This statement could explain why some genes such as Notch2 and Cdk2 exhibit an increase in trimethylation and a decrease in acetylation. More studies regarding this issue are in progress in our laboratory.
In conclusion, in the present work, we showed that i) daily exposure to low doses of 3MC during the pubertal period alters the expression of a subset of ovarian genes involved in different physiological processes such as activation of primordial follicles, cell adhesion, stress and tumor progression, apoptosis and growth factors; ii) most of these changes occur in an AhR-dependent mechanism; and iii) 3MC exposure is also able to induce epigenomic remodeling at target promoters. It is important to highlight the direct participation of AhR in inducing the changes in the gene expression and post-transcriptional modifications caused by 3MC in the rat ovary. Despite the low doses used in these experiments, daily exposure to 3MC induced both genomic and epigenomic changes which may lead to premature ovarian failure, without discarding a high risk of developing a carcinogenic process.
Supplementary Material
Acknowledgments
We thank Marcela Marquez and Enzo Cuba for their technical assistances in the maintenance and treatments of animals. This work was supported by PIP 112-2008000271 from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), UBACYT 20020130100156BA from Universidad de Buenos Aires, Argentina, and NIH: 5R01HD084542-02 from the National Institute of Health, USA.
Footnotes
Ethical standards
All animals were handled according to the Guiding Principles for the Care and Use of Research Animals, and all studies were approved by the Institutional Committee of the School of Medicine of Buenos Aires University, Argentina (CICUAL)
Conflict of interest
The authors declare they have no conflicts of interest.
References
- Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, Yasutomo K. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA. 2010;107:5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol. 2005;25:10040–10051. doi: 10.1128/MCB.25.22.10040-10051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge MG, Hutz RJ. Autoradiographic localization of aromatic hydrocarbon receptor (AHR) in rhesus monkey ovary. Am J Primatol. 2007;69:681–691. doi: 10.1002/ajp.20381. [DOI] [PubMed] [Google Scholar]
- Barnett KR, Tomic D, Gupta RK, Babus JK, Roby KF, Terranova PF, Flaws JA. The aryl hydrocarbon receptor is required for normal gonadotropin responsiveness in the mouse ovary. Toxicol Appl Pharmacol. 2007a;223:66–72. doi: 10.1016/j.taap.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett KR, Tomic D, Gupta RK, Miller KP, Meachum S, Paulose T, Flaws JA. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol Reprod. 2007b;76:1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- Beedanagari SR, Taylor RT, Bui P, Wang F, Nickerson DW, Hankinson O. Role of epigenetic mechanisms in differential regulation of the dioxin-inducible human CYP1A1 and CYP1B1 genes. Mol Pharmacol. 2010;78:608–616. doi: 10.1124/mol.110.064899.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56:382–388. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- Benedict JC, Miller KP, Lin TM, Greenfeld C, Babus JK, Peterson RE, Flaws JA. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod. 2003;68:1511–1517. doi: 10.1095/biolreprod.102.007492. [DOI] [PubMed] [Google Scholar]
- Bonello N, Jasper MJ, Norman RJ. Periovulatory expression of intercellular adhesion molecule-1 in the rat ovary. Biol Reprod. 2004;71:1384–1390. doi: 10.1095/biolreprod.104.030650. [DOI] [PubMed] [Google Scholar]
- Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167:191–198. doi: 10.1006/taap.2000.9006. [DOI] [PubMed] [Google Scholar]
- Brouwer-Visser J, Huang GS. IGF2 signaling and regulation in cancer. Cytokine Growth Factor Rev. 2015;26:371–377. doi: 10.1016/j.cytogfr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Roth R, Althoff J, Grandjean C, Patil K, Marsh S, Mclaughlin D. Carcinogenicity and metabolic profiles of 3-methylcholanthrene oxygenated derivatives at the 1 and 2 positions. Chem Biol Interact. 1978;22:69–81. doi: 10.1016/0009-2797(78)90150-3. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Heimler I, Rawlins RG, Wimpee BA, Sommer C, Hutz RJ. Estrogen receptor and aromatic hydrocarbon receptor in the primate ovary. Endocrine. 1996;5:315–321. doi: 10.1007/BF02739065. [DOI] [PubMed] [Google Scholar]
- Chau MD, Tuft R, Fogarty K, Bao ZZ. Notch signaling plays a key role in cardiac cell differentiation. Mech Dev. 2006;123:626–640. doi: 10.1016/j.mod.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Mccurdy EA, Miller BD, Lucier GW, Tritscher AM. Ovarian tumors in rats induced by chronic 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment. Cancer Res. 2000;60:5414–5419. [PubMed] [Google Scholar]
- Dere E, Lo R, Celius T, Matthews J, Zacharewski TR. Integration of genome-wide computation DRE search, AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver. BMC Genomics. 2011;12:365. doi: 10.1186/1471-2164-12-365.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Lee S, Cyr DM. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones. 2003;8:309–316. doi: 10.1379/1466-1268(2003)008<0309:mfrohf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesher JW, Horn J, Lehner AF. Carcinogenicity of 1-hydroxy-3-methylcholanthrene and its electrophilic sulfate ester 1-sulfooxy-3-methylcholanthrene in Sprague-Dawley rats. Biochem Biophys Res Commun. 1998;243:30–35. doi: 10.1006/bbrc.1997.8048. [DOI] [PubMed] [Google Scholar]
- Freour T, Masson D, Mirallie S, Jean M, Bach K, Dejoie T, Barriere P. Active smoking compromises IVF outcome and affects ovarian reserve. Reprod Biomed Online. 2008;16:96–102. doi: 10.1016/s1472-6483(10)60561-5. [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y, Mimura J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun. 2005;338:311–317. doi: 10.1016/j.bbrc.2005.08.162. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Keating AF. Impact of 7,12-dimethylbenz[a]anthracene exposure on connexin gap junction proteins in cultured rat ovaries. Toxicol Appl Pharmacol. 2014;274:209–214. doi: 10.1016/j.taap.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg JW, Foster R, Growdon WB, Verheijen RH, Rueda BR. Notch signaling in serous ovarian cancer. J Ovarian Res. 2014;7:95. doi: 10.1186/s13048-014-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, Ng DW, Li WH, Chen ZJ. Coordinated histone modifications are associated with gene expression variation within and between species. Genome Res. 2011;21:590–598. doi: 10.1101/gr.116467.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141:131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Hasan A, Fischer B. Epithelial cells in the oviduct and vagina and steroid-synthesizing cells in the rabbit ovary express AhR and ARNT. Anat Embryol (Berl) 2003;207:9–18. doi: 10.1007/s00429-003-0318-5. [DOI] [PubMed] [Google Scholar]
- Hernández-Ochoa I, Barnett-Ringgold KR, Dehlinger SL, Gupta RK, Leslie TC, Roby KF, Flaws JA. The ability of the aryl hydrocarbon receptor to regulate ovarian follicle growth and estradiol biosynthesis in mice depends on stage of sexual maturity. Biol Reprod. 2010;83:698–706. doi: 10.1095/biolreprod.110.087015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77:547–559. doi: 10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe FS, Fischl H, Murray SC, Mellor J. Is H3K4me3 instructive for transcription activation? Bioessays. 2017;39:1–12. doi: 10.1002/bies.201600095. [DOI] [PubMed] [Google Scholar]
- Huang B, Butler R, Miao Y, Dai Y, Wu W, Su W, Fujii-Kuriyama Y, Warner M, Gustafsson JÅ. Dysregulation of Notch and ERα signaling in AhR−/− male mice. Proc Natl Acad Sci USA. 2016;113:11883–11888. doi: 10.1073/pnas.1613269113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter PJ, Swanson BJ, Haendel MA, Lyons GE, Cross JC. Mrj encodes a DnaJ-related co-chaperone that is essential for murine placental development. Development. 1999;126:1247–1458. doi: 10.1242/dev.126.6.1247. [DOI] [PubMed] [Google Scholar]
- Jablonska O, Piasecka J, Ostrowska M, Sobocinska N, Wasowska B, Ciereszko RE. The expression of the aryl hydrocarbon receptor in reproductive and neuroendocrine tissues during the estrous cycle in the pig. Anim Reprod Sci. 2011;126:221–228. doi: 10.1016/j.anireprosci.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Jung NK, Park JY, Park JH, Kim SY, Park JK, Chang WK, Lee HC, Kim SW, Chun SY. Attenuation of cell cycle progression by 2,3,7,8-tetrachlorodibenzo-p-dioxin eliciting ovulatory blockade in gonadotropin-primed immature rats. Endocr J. 2010;57:863–871. doi: 10.1507/endocrj.k10e-220. [DOI] [PubMed] [Google Scholar]
- Khorram O, Garthwaite M, Golos T. Uterine and ovarian aryl hydrocarbon receptor (AHR) and aryl hydrocarbon receptor nuclear translocator (ARNT) mRNA expression in benign and malignant gynaecological conditions. Mol Hum Reprod. 2002;8:75–80. doi: 10.1093/molehr/8.1.75. [DOI] [PubMed] [Google Scholar]
- Ko CB, Kim SJ, Park C, Kim BR, Shin CH, Choi S, Chung SY, Noh JH, Jeun JH, Kim NS, Park R. Benzo(a)pyrene-induced apoptotic death of mouse hepatoma Hepa1c1c7 cells via activation of intrinsic caspase cascade and mitochondrial dysfunction. Toxicology. 2004;199:35–46. doi: 10.1016/j.tox.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Sogawa K, Fujii-Kuriyama Y. Cooperative interaction between AhR. Arnt and Sp1 for the drug-inducible expression of CYP1A1 gene. J Biol Chem. 1996;271:12310–12316. doi: 10.1074/jbc.271.21.12310. [DOI] [PubMed] [Google Scholar]
- Lawrence RJ, Earley K, Pontes O, Silva M, Chen ZJ, Neves N, Viegas W, Pikaard CS. A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell. 2004;13:599–609. doi: 10.1016/s1097-2765(04)00064-4. [DOI] [PubMed] [Google Scholar]
- Livingstone C. IGF2 and cancer. Endocr Relat Cancer. 2013;20:R321–339. doi: 10.1530/ERC-13-0231. [DOI] [PubMed] [Google Scholar]
- Lomniczi A, Wright H, Castellano JM, Sonmez K, Ojeda SR. A system biology approach to identify regulatory pathways underlying the neuroendocrine control of female puberty in rats and nonhuman primates. Horm Behav. 2013;64:175–186. doi: 10.1016/j.yhbeh.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen TM, Moriyama T, Morita Y, Perez GI, Korsmeyer SJ, Sherr DH, Tilly JL. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002;143:615–620. doi: 10.1210/endo.143.2.8624. [DOI] [PubMed] [Google Scholar]
- Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- Mattison DR. Morphology of oocyte and follicle destruction by polycyclic aromatic hydrocarbons in mice. Toxicol Appl Pharmacol. 1980;53:249–259. doi: 10.1016/0041-008x(80)90424-x. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Thorgeirsson SS. Ovarian aryl hydrocarbon hydroxylase activity and primordial oocyte toxicity of polycyclic aromatic hydrocarbons in mice. Cancer Res. 1979;39:3471–3475. [PubMed] [Google Scholar]
- Meng E, Shevde LA, Samant RS. Emerging roles and underlying molecular mechanisms of DNAJB6 in cancer. Oncotarget. 2016;7:53984–53996. doi: 10.18632/oncotarget.9803. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mitra A, Fillmore RA, Metge BJ, Rajesh M, Xi Y, King J, Ju J, Pannell L, Shevde LA, Samant RS. Large isoform of MRJ (DNAJB6) reduces malignant activity of breast cancer. Breast Cancer Res. 2008;10:R22. doi: 10.1186/bcr1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Oesterling E, Toborek M, Hennig B. Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol Appl Pharmacol. 2008;232:309–316. doi: 10.1016/j.taap.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KK, Townson DH. Prolactin-induced expression of intercellular adhesion molecule-1 and the accumulation of monocytes/macrophages during regression of the rat corpus luteum. Biol Reprod. 2000;62:1571–1578. doi: 10.1095/biolreprod62.6.1571. [DOI] [PubMed] [Google Scholar]
- Owens EO, Toborek M, Hennig B. Flavonoids protect against intercellular adhesion molecule-1 induction by benzo[a]pyrene. Bull Environ Contam Toxicol. 2009;83:4–7. doi: 10.1007/s00128-009-9664-1. [DOI] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P, Liu K. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol. 2007;21:289–202. doi: 10.1210/me.2007-0172. [DOI] [PubMed] [Google Scholar]
- Rhon-Calderón EA, Galarza RA, Lomniczi A, Faletti AG. The systemic and gonadal toxicity of 3-methylcholanthrene is prevented by daily administration of α-naphthoflavone. Toxicology. 2016;353–354:58–69. doi: 10.1016/j.tox.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Robles R, Morita Y, Mann KK, Perez GI, Yang S, Matikainen T, Sherr DH, Tilly JL. The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology. 2000;141:450–453. doi: 10.1210/endo.141.1.7374. [DOI] [PubMed] [Google Scholar]
- Sadeu JC, Foster WG. The cigarette smoke constituent benzo[a]pyrene disrupts metabolic enzyme, and apoptosis pathway member gene expression in ovarian follicles. Reprod Toxicol. 2013;40:52–59. doi: 10.1016/j.reprotox.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Schnekenburger M, Peng L, Puga A. HDAC1 bound to the Cyp1a1 promoter blocks histone acetylation associated with Ah receptor-mediated trans-activation. Biochim Biophys Acta. 2007;1769:569–578. doi: 10.1016/j.bbaexp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- Shiromizu K, Mattison DR. Murine oocyte destruction following intraovarian treatment with 3-methylcholanthrene or 7,12-dimethylbenz(a)anthracene: protection by alpha-naphthoflavone. Teratog Carcinog Mutagen. 1985;5:463–472. doi: 10.1002/tcm.1770050609. [DOI] [PubMed] [Google Scholar]
- Sims P. Qualitative and quantitative studies on the metabolism of a series of aromatic hydrocarbons by rat-liver preparations. Biochem Pharmacol. 1970;19:795–818. doi: 10.1016/0006-2952(70)90243-1. [DOI] [PubMed] [Google Scholar]
- Sims P. The metabolism of 3-methylcholanthrene and some related compounds by rat-liver homogenates. Biochem J. 1966;98:215–228. doi: 10.1042/bj0980215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobinoff AP, Beckett EL, Jarnicki AG, Sutherland JM, McCluskey A, Hansbro PM, McLaughlin EA. Scrambled and fried: cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. Toxicol Appl Pharmacol. 2013;271:156–167. doi: 10.1016/j.taap.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the Villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci. 2011;123:563–575. doi: 10.1093/toxsci/kfr195. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Nixon B, Roman SD, McLaughlin EA. Staying alive: PI3K pathway promotes primordial follicle activation and survival in response to 3MC-induced ovotoxicity. Toxicol Sci. 2012a;128:258–271. doi: 10.1093/toxsci/kfs137. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Pye V, Nixon B, Roman SD, McLaughlin EA. Jumping the gun: smoking constituent BaP causes premature primordial follicle activation and impairs oocyte fusibility through oxidative stress. Toxicol Appl Pharmacol. 2012b;260:70–80. doi: 10.1016/j.taap.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Sun L, Tan L, Yang F, Luo Y, Li X, Deng HW, Dvornyk V. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2012;19:126–132. doi: 10.1097/gme.0b013e318224f9ac. [DOI] [PubMed] [Google Scholar]
- Tang T, Lin X, Yang H, Zhou L, Wang Z, Shan G, Guo Z. Overexpression of antioxidant enzymes upregulates aryl hydrocarbon receptor expression via increased Sp1 DNA-binding activity. Free Radic Biol Med. 2010;49:487–492. doi: 10.1016/j.freeradbiomed.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KE, Bourguet SM, Christian PJ, Benedict JC, Sipes IG, Flaws JA, Hoyer PB. Differences between rats and mice in the involvement of the aryl hydrocarbon receptor in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss. Toxicol Appl Pharmacol. 2005;203:114–123. doi: 10.1016/j.taap.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Kietz S, Ström A, Gustafsson JA. HES-1, a novel target gene for the aryl hydrocarbon receptor. Mol Pharmacol. 2004;65:165–171. doi: 10.1124/mol.65.1.165. [DOI] [PubMed] [Google Scholar]
- Vanorny DA, Mayo KE. The role of Notch signaling in the mammalian ovary. Reproduction. 2017;153:R187–R204. doi: 10.1530/REP-16-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol. 2014;28:499–511. doi: 10.1210/me.2013-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò P, Fusi F, Gaffuri B, Bonzi V, Ferrari A, Vignali M. Soluble intercellular adhesion molecule-1 in ovarian follicles: production by granulosa luteal cells and levels in follicular fluid. Fertil Steril. 1998;69:774–779. doi: 10.1016/s0015-0282(97)00565-7. [DOI] [PubMed] [Google Scholar]
- Viganò P, Gaffuri B, Ragni G, Di Blasio AM, Vignali M. Intercellular adhesion molecule-1 is expressed on human granulosa cells and mediates their binding to lymphoid cells. J Clin Endocrinol Metab. 1997;82:101–105. doi: 10.1210/jcem.82.1.3646. [DOI] [PubMed] [Google Scholar]
- Xia M, Viera-Hutchins L, Garcia-Lloret M, Noval Rivas M, Wise P, McGhee SA, Chatila ZK, Daher N, Sioutas C, Chatila TA. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J Allergy Clin Immunol. 2015;136:441–453. doi: 10.1016/j.jaci.2015.02.014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Cheng Z, Chen X, Lobe CG, Liu J. The role of Notch signalling in ovarian angiogenesis. J Ovarian Res. 2017;10:13. doi: 10.1186/s13048-017-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TT, Jiang YY, Shang L, Shi ZZ, Liang JW, Wang Z, Zhang Y, Hao JJ, Jia XM, Xu X, Cai Y, Zhan QM, Wang MR. Overexpression of DNAJB6 promotes colorectal cancer cell invasion through an IQGAP1/ERK-dependent signaling pathway. Mol Carcinog. 2015;54:1205–1213. doi: 10.1002/mc.22194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


