Abstract
Although it has become clear that sexual selection may shape mating systems and drive speciation, the potential constraints of environmental factors on processes and outcomes of sexual selection are largely unexplored. Here, we investigate the geographic variation of such environmental factors, more precisely the quality and quantity of nest resources (bivalve shells) along a salinity gradient in the Baltic Sea Area (Baltic Sea, Sounds and Belts, and Kattegat). We further test whether we find any salinity-associated morphological differences in body size between populations of common gobies Pomatoschistus microps, a small marine fish with a resource-based mating system. In a geographically expansive field study, we sampled 5 populations of P. microps occurring along the salinity gradient (decreasing from West to East) in the Baltic Sea Area over 3 consecutive years. Nest resource quantity and quality decreased from West to East, and a correlation between mussel size and male body size was detected. Population density, sex ratios, mating- and reproductive success as well as brood characteristics also differed between populations but with a less clear relation to salinity. With this field study we shed light on geographic variation of distinct environmental parameters possibly acting on population differentiation. We provide insights on relevant ecological variation, and draw attention to its importance in the framework of context-dependent plasticity of sexual selection.
Keywords: aquatic ecology, body size, environmental gradient, nest availability, nesting resources, sexual selection
Many aspects of an organism’s social organization and mating system can be predicted if the characteristics of its environment are known. Environmental factors can, for example, determine to which degree mates or resources can be defended and monopolized, and such ecological constraints impose limits on the degree to which sexual selection can operate (Emlen and Oring 1977; Forsgren et al. 1996a; Gillespie et al. 2014). While it is well known that sexual selection is influenced by environmental factors (Hill 1994; Kwiatkowski and Sullivan 2002; Gamble et al. 2003; Cornwallis and Uller 2010; Gillespie et al. 2014), surprisingly little attention has been paid to environmentally dependent differences in the plasticity of reproductive decisions. Specifically, differences in the environmental context may cause variation of sexual selection over space or time (Gwynne and Simmons 1990; Almada et al. 1995; Forsgren et al. 1996a; Siepielski et al. 2009; Janicke et al. 2015; Monteiro et al. 2017).
Mating systems and sex-role dynamics are strongly tied to processes and outcomes of sexual selection (Emlen and Oring 1977; Kvarnemo and Ahnesjö 1996), and sexual selection theory predicts strong selection for traits that increase reproductive success (Jennions and Kokko 2010; Kokko et al. 2012). For example, an individual’s access to mates and resources within a population depends on its competitive ability (Parker and Sutherland 1986). Male competitive ability generally increases with body size, because larger males are often more successful in monopolizing resources necessary for mating, either directly by monopolizing females or indirectly by monopolizing nest sites (Andersson 1994). Thus, larger males are often favored by sexual selection, and are generally selected for via male contest or via female choice (Bonduriansky and Rowe 2003; Lindström and Pampoulie 2005; Dubey et al. 2009; Wacker et al. 2014).
Body size does not only vary within populations due to intra- or inter-sexual selection favoring specific phenotypes but can also vary strongly among populations due to natural selection (Rundle et al. 2006). For example, it has been shown that body size often either increases with increasing latitude (Bergmann’s rule, Bergmann 1847) or decreases with increasing latitude (converse Bergmann’s rule; Lindsey 1966; Murphy 1985; Blackburn et al. 1999; Stillwell et al. 2007). However, there are also examples for an absence of macroecological body size relationships (Rypel 2014). Moreover, body size variation among populations has also been found along other environmental gradients (e.g., temperature, precipitation, water depth, salinity; Smith and Brown 2002; Westerbom et al. 2002; Collins et al. 2005; Liao and Lu 2011). Thus, phenotypic variation in growth, body mass, or absolute size may arise as a consequence of environmental conditions or due to sexual selection, and the effects may counteract one another (Janicke et al. 2015; Monteiro et al. 2017). Due to the physiological stress of osmoregulation (Evans and Claiborne 2008), we predict body size to decrease with decreasing salinity. The interaction between natural and sexual selection may, therefore, vary among populations as a function of environmental heterogeneity.
Environmental gradients allow us to examine linked patterns of adaptations and changes in selection pressures, mating systems, and life-histories (Hargreaves and Eckert 2014). The Baltic Sea Area (HELCOM 1992) constitutes such an extreme environment with a steep decrease in salinity from West (25 Practical Salinity Units [PSU]) to East (1–2 PSU) and many species find their distribution limits over these salinity gradients (e.g., Jansen et al. 2009). In addition to spatial variation of salinity, surface water temperature along the coast of the Baltic Sea Area also shows a latitudinal and longitudinal gradient (HELCOM 1993, 1996; Ojaveer et al. 2010). Hence, a seasonal surface water temperature gradient exists, with water temperatures rising later in the year in the North and East than in the South and West, which may affect for instance the duration of breeding cycles and egg development (St Mary et al. 2004), fecundity and lifespan (Kim et al. 2017), and the strength of sexual selection itself (Monteiro and Lyons 2012). Habitats in the Baltic Sea Area can differ greatly in nesting resource availability (Forsgren et al. 1996a), thus the cost of reproduction can differ between populations and consequently affect potential reproductive rates of both sexes (Ahnesjö et al. 2001). Furthermore, different levels of resource competition may interact with mate competition in its effects on sexual selection (Wacker and Amundsen 2014).
Differences in salinity along the ecological gradient in the Baltic Sea Area can act both directly on fitness by affecting an organism’s metabolism and population growth rate (Evans and Claiborne 2008), as well as indirectly by limiting resources necessary for reproduction. For example, common goby males Pomatoschistus microps frequently use blue mussels Mytilus edulis or cockles (Cerastoderma edule and Cerastodermaglaucum) as nest substrate. However, these bivalves are less tolerant to low salinity. As a result, blue mussels show a significant decrease in biomass from West to East (Westerbom et al. 2002), while C. edule does not even extend into waters with salinities below 10–11 PSU (Brock 1980). For a better understanding of the strength and direction of sexual selection, it is necessary to know the degree of variation in nest availability between populations in common gobies. We predict a decrease in the overall nest availability and the occurrence of smaller, more fragile soft-shell clams Mya arenaria with decreasing salinity from West to East in the Baltic Sea Area. This would imply consequences for the sexual selection regime among P. microps populations along the salinity gradient, with higher competition over nests (i.e., intra-sexual competition) in the East than in the West.
Not only nest availability but also demographic factors like population density and sex ratios influence the degree and direction of competition in a population (Kokko and Rankin 2006; Kokko and Jennions 2008). Thus, we estimated population density as well as sex ratios (adult sex ratio [ASR] and operational sex ratio [OSR]) representing the ratio of adult individuals and the ratio of ready-to-mate individuals in a population, respectively, in 5 populations along the salinity gradient of the Baltic Sea Area. Population density is expected to be highest with intermediate salinity, which is known to lead to increased growth rates and reproductive success in several marine fish species (Boeuf and Payan 2001). The OSR may be strongly affected by nest availability with for instance a shortage of bivalve shells as nesting resources allowing not all males to provide a nest for mating, and thus may be female biased due to few nest holding males being ready-to-mate (Forsgren et al. 2004). In nature, variation in quantity and quality of nesting resources may covary and be confounded by other temporally or spatially varying biotic or abiotic factors that may influence the mating system and sexual selection (Emlen and Oring 1977; Forsgren et al. 1996a). Therefore, using standardized quantities and qualities of nesting resources (Lindström 1988), we conducted a mating assay to estimate mating- and reproductive success as well as differences in brood characteristics between populations. Variation in reproductive success is expected to reveal differences in the sexual selection mode between populations.
With the focus on linking spatially varying environmental factors to demographic and phenotypic differences between populations, we aim to provide evidence for the fundamental importance to consider the underlying environmental context in further studies on context-dependent plasticity of sexual selection. The main objectives of our study were to investigate how (1) quantity and quality of nesting resources, (2) demographic factors, and (3) body size vary between populations of P. microps along a salinity gradient in the Baltic Sea Area and possibly affect (4) mating- and reproductive success. According to our objectives, we predict (1) a decrease in nest availability and the occurrence of smaller, more fragile soft-shell clams (M. arenaria) with decreasing salinity from West to East in the Baltic Sea Area. (2) Population density is expected to be highest at increased growth rates and reproductive success at intermediate salinity. (3) We predict body size to decrease with decreasing salinity, and expect (4) variation in reproductive success among populations to act in concert with salinity-driven differences in nest competition, that is, intra-sexual competition being higher in the East than in the West.
Materials and Methods
Study species
The common goby P. microps is a small euryhaline, benthic, annual fish species with a resource-based mating system. It reproduces repeatedly during a single breeding season between May and August during which males compete over nest structures such as mussel shells and rocks, attract females by courtship displays, and provide exclusive paternal care for the brood after spawning (Nyman 1953; Borg et al. 2002). Pomatoschistus microps has a promiscuous mating system where males can care for eggs from several females simultaneously and females spawn with different males (Miller 1975). The common goby copes with a wide range of conditions due to being the most temperature and salinity tolerant species within the sand goby group (Fonds and van Buurt 1974) and occurs along the European Atlantic coast, at 2 populations in the Mediterranean, and in the Baltic Sea Area, where it inhabits marine, brackish, and extremely brackish habitats that exist within a relatively small geographic range (Fonds and van Buurt 1974).
Field study design
All data collected are from a field-based study conducted over several years (2012–2014) in order to provide insights into how geographic variation in nesting resources, population density, sex ratios, and body size in a marine fish along an environmental gradient may shift prospects for mating. In a combined effort of habitat surveys and population sampling of 5 P. microps populations along the salinity gradient in the Baltic Sea Area (Figure 1), we repeatedly visited sites early and late during the breeding season (Table 1). We collected data on several variables: (1) salinity and temperature of the water were measured at all sampling sites multiple times using a HACH multi-probe (HACH Lange GmbH; Table 1), (2) quantity and quality of nesting resources, (3) number of encountered males and females as a proxy for population density, as well as sex ratios (ASR and OSR), (4) body size of P. microps, and (5) variation in mating- and reproductive success as well as properties of broods.
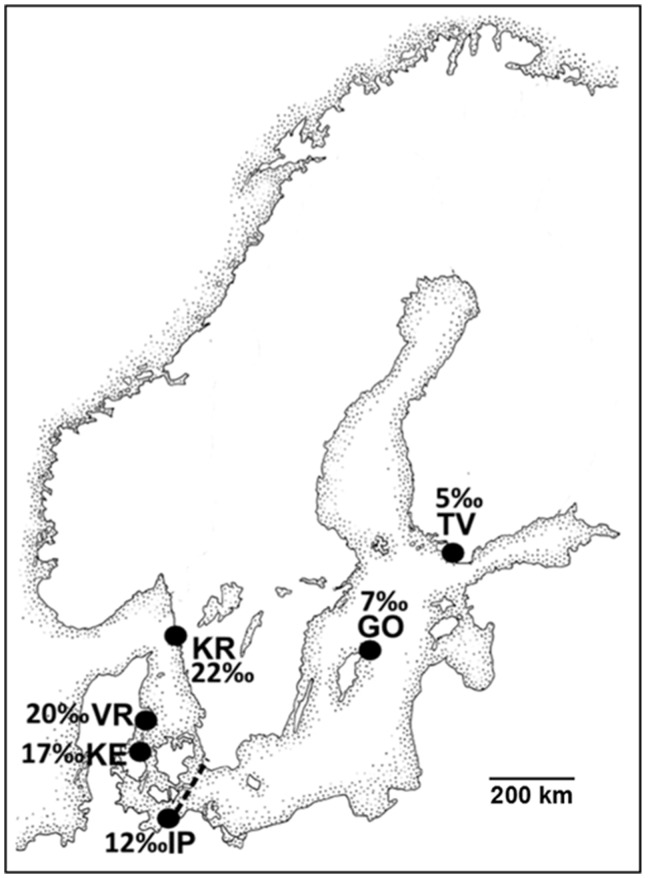
Figure 1.
The Baltic Sea Area, including the Baltic Sea and the entrance to the Baltic Sea (HELCOM 1992). Shown are the 6 sampling sites of this study (TV, GO, IP) within the Baltic Sea proper, (KE) in the Sounds and Belts region, and (VR, KR) in the Kattegat. For each site, local average salinity (PSU) measured during data collection in 2012, 2013, and 2014 is included. The dashed line Falsterbo (south Sweden)–Travemünde (Germany) is marking the entrance of the Baltic Sea proper. See Table 1 for site abbreviations. Map modified after Forsgren et al. (1996a).
Table 1.
Sampling sites in the Baltic Sea Area where data on habitat, population density, and nest substrate were collected
| Sampling site | Geographic location | ICES | Abb. | Salinity | Salinity class | Temp. (°C) |
Latitude | Longitude | 2012 |
2013 |
2014 |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSU | Early | Late | Early | Late | Early | Late | Early | Late | |||||||
| Kristineberg | Bohuslän, Western Sweden | 23 | KR | 22 | High | 16.1 | 19.1 | 58°24′ N | 11°46′ E | T/S/A | T/S | T | T/S/M | ||
| Vrinners | Jutland, Kaløvig, Denmark | 22 | VR | 20 | Intermediate | 19.6 | 50°14′ N | 10°30′ E | A | ||||||
| Kerteminde | Funen, Great Belt, Denmark | 22 | KE | 17 | Intermediate | 18.1 | 19.0 | 55°44′ N | 10°65′ E | T/S/A | T/S/M | ||||
| Poel Island | Bay of Mecklenburg, Germany | 22 | IP | 12 | Intermediate | 15.1 | 18.2 | 53°99′ N | 11°48′ E | T/S/A | T/S | T/S | T/S/M | T | |
| Gotland | Gotland Basin, Sweden | 28 | GO | 7 | Low | 16.6 | 17.5 | 57°78′ N | 18°94′ E | T/S/A | T/S | T/S | S/M | T | |
| Tvärminne | Archipelago, Southern Finland | 29 | TV | 5 | Low | 13.5 | 15.2 | 59°82′ N | 23°14′ E | T/S/A | T/S | T/S | T/M | T/S | |
Notes: Indicated are: sampling sites, geographic locations, international council for the exploration of the sea (ICES) subdivisions, abbreviations for sites (Abb.), salinity (PSU) and salinity classes, mean surface water temperature pooled over 3 consecutive years (2012–2014) early and late during the breeding season, and coordinates (latitude and longitude). Data collection of different variables vary between populations, year, and time points during the breeding season: T (transect data collected), S (body size measurements), M (morphometric analyses), and A (mating assay conducted). Blanks denote missing data.
Habitat survey
Each sampling site was visited between 2012 and 2014 2–5 times both early and late in the breeding season (Table 1, sites marked with T). We defined the early breeding season from the beginning of May to mid-June and the late breeding season from late June to late July. Visits were organized as much as logistically possible to follow the phenology of the beginning of breeding season based on different water temperatures and sea ice (HELCOM 1993; Peck et al. 2012) by traveling among sites from West to East and from South to North and keeping the time interval and order of visits constant between early and late visits. Habitat surveys were conducted by snorkelling 2 different transects during each visit at the 5 sites along a 20-m lead line in shallow water (< 70 cm depth) colonized by common gobies. We collected data on nesting resource availability, population density, and sex ratios within ca. 50 cm of each side of the lead line. To estimate the OSR, males occupying a nest were categorized as males ready-to-mate and females were categorized according to the roundness of their bellies indicating readiness to spawn (Borg et al. 2002). We divided the females into 3 ripeness stages (R1–R3; Figure 2): R1: unripe (no ripe eggs, “slim” sensuBorg et al. 2002), R2: ripe (slightly rounded abdomen, some ripe eggs), R3: late ripeness (the often brightly orange-pinkish abdomen is extremely round with a tadpole-like shape and completely filled with eggs) whereby only females of R2 and R3 were counted as ready-to-mate (“gravid” sensuBorg et al. 2002) for assessing the OSR (Figure 2). Data collected along the 2 20 m transects per site and visit were pooled, because of very low encountered densities at low salinity sites. For easier interpretation of the results, populations of the selected sites were categorized based on ICES (Table 1) and salinity (PSU) in: high (KR: 22.2), intermediate (KE: 16.8/VR: 20.0 and IP: 12.2), and low salinity habitats (GO: 7.2 and TV: 5.4).
Figure 2.

Female common gobies representing the 3 ripeness stages to which they were assigned to by visual inspection, from bottom-up: R1: unripe, no eggs in belly, slim; R2: ripe, some eggs in belly, gravid-; R3: late ripeness, belly extremely filled with eggs, gravid+.
Nesting resources
The number of available nesting resources (including empty shells of M. arenaria, M. edulis, Cerastoderma sp.), as well as the number of natural nests occupied by males was counted along transects. To compare natural nest size variation between populations, we measured the diameter of randomly sampled M. arenaria shells used as nests. Mya arenaria is the only bivalve shell that occurs at all sampled sites, including those with low salinity, and exhibits a size range that qualifies as potential nests for common gobies (Strasser 1999; this study). Mussel nests were collected during the early breeding season, except for IP where mussel nests were collected in the early and late breeding season. Nests were collected in 2 consecutive years (2013–2014; exception: KE only 2014).
Population density and sex ratios
Our data on individual counts (population density) along transects include females of the 3 ripeness stages, freely swimming males, and males occupying a nest. Individual male and female count data can be used to calculate the ASR (calculated as the fraction of adult males to all adult individuals), because typically only adult individuals are present from May to July. The OSR was calculated as the fraction of all mating-ready males (males occupying a nest) to all mating-ready individuals (Kvarnemo and Ahnesjö 1996), where only females in stages R2 and R3 (gravid) were included.
Body size
We measured body size of individuals along the salinity gradient as a possible phenotypic trait being directly affected by geographic variation in salinity. Fish of both sexes were caught in shallow waters near the coast at 5 sampling sites using hand trawls (always the first 25 individuals caught of each sex were used for analysis) for body size measurements (Table 1, sites marked with S). We measured the total length (TL) to the nearest 1 mm. Whenever possible, measurements of 25 individuals of each sex were used (with some exceptions in 2013: GO early: females: n = 20, males: n = 24, GO late: females: n = 6; TV early: males: n = 21 and late: n = 18). After measurements were taken, fish were released back to their natural habitat.
Mating assay
In order to gather data on (a) nest colonization, (b) mating success, (c) reproductive success, and (d) properties of broods, an assay with standardized nest availability (30), exposure (72 h), and quality was carried out in 2012 at 5 different sites in the Baltic Sea Area (KR, VR, IP, GO, and TV; for details see Table 1, sites marked with A). Because of logistic reasons, the field assay was not conducted at KE but instead at VR, a population ca. 80 km further north in the Kattegat, with similar ecological conditions and habitat structure but slightly higher salinity (20 PSU; for details, see Table 1 and Figure 1). Thirty ceramic tiles (4 × 4 cm), readily accepted as nest resource by common goby males, were put out in shallow water (< 70 cm depth). After 72 h we checked each nest for nest occupation by a resident male and collected all tiles and photographed broods if the male received eggs. Male reproductive success was estimated by the brood-area (mm2), which was measured using ImageJ version 1.47 (W. Rasband, National Institute of Health, USA). Even though it is common for males to cannibalize on eggs (Vallon and Heubel 2016; Vallon et al 2016a), it is possible to see where eggs had been attached to the tile prior to cannibalism or egg predation due to visible residues of mucus (authors’ personal observation). This method thus allowed us to measure the initial brood area, even when at the time of nest retrieval some eggs had been removed by either filial cannibalism or egg predation. It is difficult to judge whether egg removal had been due to filial cannibalism or egg predation by solely looking at the residuals on the ceramic tiles. However, as in all observed cases of egg removal nests appeared undisturbed and intact and were still maintained by the male, we assume filial cannibalism as the more likely reason for brood reduction. To make sure that our measure of reproductive success was not influenced by differences in the density or size of the eggs, the number of eggs within a 0.5 × 0.5 cm2 square as well as the size of these counted eggs were measured using ImageJ (number of broods: KR, VR, GO: n = 4 and IP, TV: n = 3). Since only 1 male received eggs at TV within 72 h, this population was excluded from analyses of reproductive success. Here, measures of egg density and egg size were collected from the 2 successful (out of 30) artificial nests that were exposed for an extended period of 92 h.
Data analysis
Statistical procedures
Data on mating resources, population density, sex ratios, body size, and reproductive success as well as brood characteristics collected over consecutive years (2012–2014) were centered around the yearly mean to account for differences between years. Normality of variables was checked via visual inspection of residuals and q–q normality plots. To achieve normality, count data were log-transformed (log10; “Available nests”) and proportion data (“Proportion of occupied nests” and “Sex ratios”) were arcsine-transformed (asn) using as suggested by Zar (1984).
We conducted several linear models (LMs) using R (R Core Team 2012) with centered data as outcome variable and “population” set as factors ranked according to salinity (from West: high to East: low) as well as “season” (early and late during the breeding season) as independent variables. Non-significant interactions “population:season” were excluded from the model. If a significant interaction with “season” was found, the data set was divided into “early” and “late” and analyzed for both levels within the breeding season separately. Pairwise post hoc comparisons between populations were conducted controlling for the false discovery rate (at level α).
Male body size and M. arenaria shell length
Mean clam shell length (mm TL) of M. arenaria nests and mean male body size (mm TL) for each sampling event (i.e., same site, year, season, n = 9) were correlated using a Spearman’s rank correlation rho (ρ).
Mating assay
For the binomial response variables in the mating assay on nest occupation and mating success after 72 h of each artificial nest (n = 30 per site), frequencies of occurrence (frequency of success and failure) were used. To test if observed values differed from expected values (based on either the average proportion of nest occupation across all populations or, for mating success, on the population-specific proportion of nest occupation), we used contingency table analyses with log-likelihood ratio tests with Yates correction.
Results
Nesting resources
Nest quantity
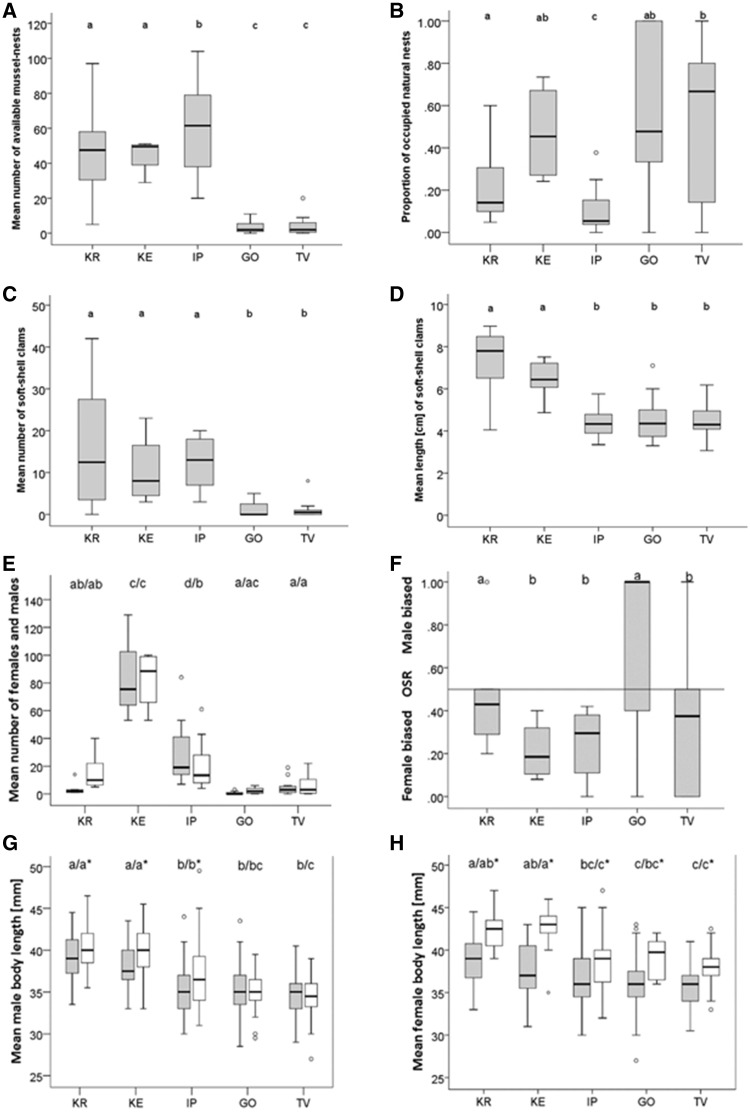
Low salinity sites (GO and TV) showed a much lower number of available nests than high (KR) or intermediate (KE, IP) salinity sites (Figure 3A). The availability of natural nests (M. arenaria, M. edulis, C. edule, and C. glaucum) along the salinity gradient differed significantly between populations (but not between early and late within the breeding season; Table 2).
Figure 3.
Data collected along transects (2 × 20 m) over 3 consecutive breeding seasons during the years 2012–2014. (A) Mean number of available nest resources (free and occupied), (B) proportion of natural nests occupied, and the (C) number of soft-shell clams M. arenaria as well as their (D) mean length [cm]. (E) The number of female (gray boxes) and male (white boxes) common gobies counted (population density) and (F) the OSR (the fraction of all ready-to-mate males to all ready-to-mate individuals). (G and H) The mean centered body length (TL mm) for (G) males and (H) females measured early (gray boxes) and late (empty boxes) during the breeding season. Box plots represent the medians and the first and third quartiles. Whiskers represent the most extreme data point ≤ 1.5 times the interquartile range from the box. Outliers are shown as separate data points. Letters above box plots indicate significant differences between populations (same letters denote no significant differences). The total number of transects between populations varies, for details and site abbreviations see Table 1. Note that for easier interpretation, untransformed non-standardized data are shown. Significances refer to centered (Panels A, C, and D) and transformed [A (log10), B (asn)] data (see also Table 2). Asterisks denote significant differences between early and late season within populations in Panels G and H.
Table 2.
Results of final LMs for all variables tested
| Group | Variable | Variation | Final model |
Pop |
Season |
Sea * Pop |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trans. | Adj. R2 (%) | df | F | df | F | df | F | df | F | ||
| Mating resources | Available nests | log | 77 | 5,35 | 28.2** | 4,35 | 34.9** | 1,35 | 1.1 | NS | NS |
| Proportion of occupied nests | asn | 46 | 5,35 | 7.8** | 4,35 | 9.4** | 1,35 | 1.5 | NS | NS | |
| Soft-shell clams | 37 | 4,36 | 6.9** | 4,36 | 6.9** | ||||||
| Size soft-shell clams | 60 | 4,83 | 33.5** | 4,83 | 33.5** | ||||||
| Body size | Female | 32 | 5,294 | 28.6** | 4,294 | 22.3** | 1,294 | 53.6** | NS | NS | |
| Male | 27 | 5,294 | 22.8** | 4,294 | 28.0** | 1,294 | 1.9 | NS | NS | ||
| Densities and sex ratios | Number of females | 70 | 5,35 | 20.2** | 4,35 | 23.5** | 1,35 | 6.6* | NS | NS | |
| Number of males | 69 | 5,35 | 18.6** | 4,35 | 22.4** | 1,35 | 3.6 | NS | NS | ||
| ASR | asn | 20 | 5,35 | 3.0* | 4,35 | 3.5* | 1,35 | 0.9 | NS | NS | |
| Number of ready-to-mate females | 70 | 5,35 | 19.8** | 4,35 | 23.3** | 1,35 | 5.9* | NS | NS | ||
| Number of ready-to-mate males | 50 | 9,31 | 5.5* | 4,31 | 9.0** | 1,31 | 0.9 | 4,31 | 3.1* | ||
| OSR | asn | 37 | 5,35 | 5.6* | 4,35 | 6.0* | 1,35 | 3.9 | NS | NS | |
| Mating assay | Brood size | 18 | 4,44 | 3.7* | 4,44 | 3.7* | |||||
| Egg size | 64 | 4,761 | 338.1** | 4,761 | 338.1** | ||||||
| Egg density | 42 | 4,13 | 4.1* | 4,13 | 4.2* | ||||||
Notes: Given are groups, the specific variables tested and how variables were transformed [trans. log (log10) or asn (arcsine)] to achieve normality; the variation that is explained by the model in % (adjusted R2), the fit of the final model; the F-statistic for the independent variables “population” (pop, six sites) and “season” (early vs. late during the breeding season), and the F-statistic for the interaction term. Significant results are denoted in bold with **(P < 0.0001) and *(P < 0.05). Non-significant results are denoted with NS, empty rows denote “not tested.”
Proportion of occupied nests
The intermediate site IP, with the highest number of nests available, showed the overall lowest proportion of mussel nests taken up by males (Figure 3B). The proportion of occupied natural nests at the low salinity sites (GO and TV) showed a trend to be highest, but did not differ significantly from the intermediate site KE (Figure 3B). The site with the highest salinity (KR) showed a significantly lower proportion of occupied nests than the site with the lowest salinity (TV). The proportion of occupied natural nests differed significantly between populations (but not within breeding seasons; Table 2).
Quantity and quality of soft-shell clams (M. arenaria)
The availability of M. arenaria was significantly higher in high and intermediate salinity sites than in low salinity sites (Table 2 and Figure 3C). The size distribution of M. arenaria showed a clear separation between the 2 highest (KR and KE, laying west of the entrance into the Baltic Sea proper, Figure 1) and 3 lower salinity sites (IP, GO, and TV, laying east of entrance, within the Baltic Sea proper, Figures 1 and 3D and Table 2).
Densities and sex ratios
Population density
The number of males and females encountered along transects differed between populations (Table 2) and was highest at the intermediate sites, and especially so in KE (mean number of individuals ± SE: KE: males: 82.5 ± 10.9 and females: 83.3 ± 16.1 compared with males: 11.3 ± 2.2 and females: 10.2 ± 2.8 from KE, IP, GO, and TV; Figure 3E). Season (early vs. late during the breeding season) also had an effect on population density (Table 2), which was overall higher early in the breeding season (males: 22.3 ± 6.0, females: 23.8 ± 8.3) than in the late breeding season (males: 14.7 ± 5.4, females: 11.7 ± 4.2). We also found significant differences in population density between early and late breeding season within populations (for females in: KE: F1,31 = 8.3, P = 0.007 and IP: F1,31 = 9.3, P = 0.005; for males only in: IP: F1,31 = 4.7, P = 0.038; Table 2).
Operational sex ratio
The OSR was significantly different between populations (Table 2). The low salinity site GO was significantly more male biased than all other populations except KR (Figure 3F). The number of ready-to-mate males showed a significant interaction between population and breeding season (variation among populations early: 53%, F4,14 = 6.0, P = 0.005; late: 38%, F4,17 = 4.2, P = 0.016; Table 2). More ready-to-mate males were counted early than late in the breeding season at the 2 sites with the highest salinity (mean number of ready-to-mate males ± SE: KR: early: 13.5 ± 8.9, late: 5.5 ± 1.4; KE: early: 33.5 ± 2.5, late: 11.0 ± 4.0), which also showed overall highest number of ready-to-mate males. At the intermediate site IP (early: 2.0 ± 1.2, late: 7.8 ± 2.5) and the low salinity site GO (early: 1.7 ± 1.2, late: 2.0 ± 1.1), however, more ready-to-mate males were counted late in the breeding season than early in the breeding season. For ready-to-mate females no significant interaction between population and breeding season was detected (Table 2).
Body size
Males as well as females showed a significant decrease in body size with decreasing salinity from West to East (Table 2 and Figure 3G, H). Females of the high salinity site KR (mean body size in mm ± SE: 39.8 ± 0.30) were 1.5 mm longer than females of the intermediate sites KE and IP (38.3 ± 0.27) and 3.4 mm longer than females of the low salinity sites GO and TV (36.4 ± 0.19). Males of the high salinity site KR (39.5 ± 0.28) were 2.5 mm longer than males of the intermediate sites KE and IP (37.0 ± 0.27) and even 4.9 mm longer than males of the low salinity sites GO and TV (34.6 ± 0.20).
We found an association between breeding season and body size. Females in particular were found to be larger late in the breeding season (Table 2). For all populations showing body growth over the course of the breeding season (for males only populations KR, KE, IP), females were about 3 mm longer late in the breeding season (early: 36.9 ± 0.18, late: 39.9 ± 0.23), while males only grew 0.6 mm (early: 37.6 ± 0.27, late: 38.2 ± 0.33). Males of low salinity sites GO and TV did not grow over the breeding season (Table 2 and Figure 3H).
Male body size and size of M. arenaria
Mean male body size showed a strong positive correlation with the size of M. arenaria mussels used as nests at all 5 populations (Spearman’s rank correlation: ρ = 0.85, P = 0.006; Figure 4). The high salinity site KR showed the largest M. arenaria as well as the largest males. Following the salinity gradient both shell and male sizes decreased. The site with the lowest salinity (TV) showed also the smallest mussel nests as well as males. Natural nests with M. arenaria shells as nest substrate were at the high salinity site KR about 30 mm larger (74.4 ± 4.7 mm) than at the low salinity site TV (44.5 ± 1.9 mm; Figure 4).
Figure 4.
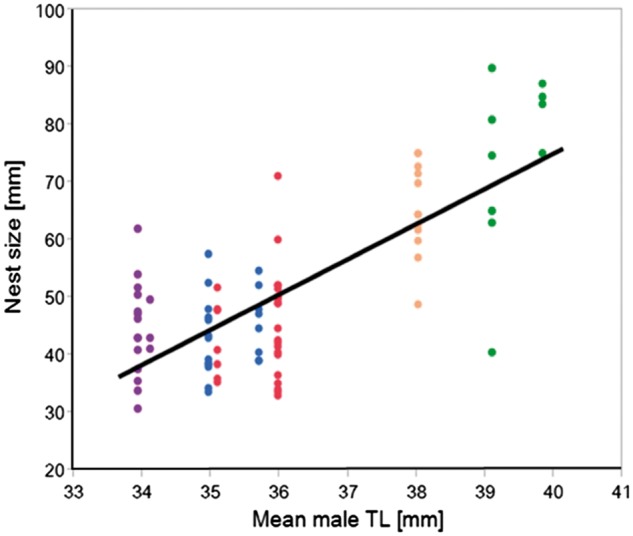
The size (mm) of clam nests M. arenaria (Mya) correlated with male mean body size (TL mm) of each site sampled in the corresponding year and time point during the breeding season (black line indicates linear regression fit). Nest clams from the high salinity site KR (green), the intermediate salinity sites KE (orange), and IP (blue) and the low salinity sites GO (red), and TV (purple) were collected during the early breeding season. Clam nests at IP were also collected late during the breeding season. Shells at all sites were collected in 2013 and 2014 except IP and KE, which were only sampled in 2014.
Mating assay
Nest occupation
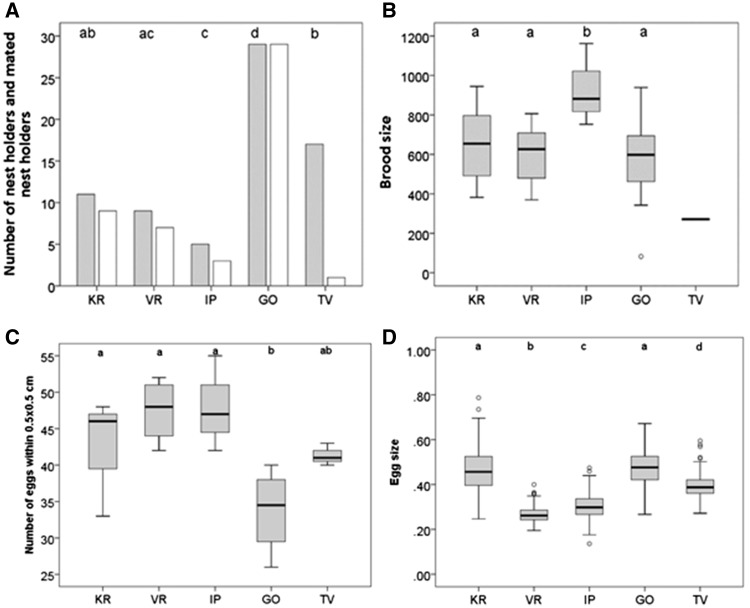
The nest occupation rate of the 30 artificial nests after 72 h experimental exposure varied significantly between the 5 populations (log-likelihood test: χ2 = 47.8, df = 4, P < 0.0001, n = 150; Figure 5A, gray bars). Testing against the overall average expected occupation rate across all populations revealed that the number of occupied nests at the high (KR: χ2 = 0.5, df = 1, P = 0.487, n = 12), the intermediate site VR (χ2 = 3.2, df = 1, P = 0.073, n = 9), as well as at the low salinity site TV (χ2 = 0.6, df = 1, P = 0.443, n = 17) did not significantly differ from the expected number of males occupying nests. The intermediate site IP however showed a significantly lower nest occupation rate than expected (χ2 = 10.6, df = 1, P = 0.001, n = 5), while the low salinity site GO showed a significantly higher rate of occupied nests than expected (χ2 = 26.6, df = 1, P < 0.0001, n = 29).
Figure 5.
Results of the mating assay after exposing 30 artificial nests for 72 h at 5 different sites in the field. (A) The number of nests occupied by nest holders (gray boxes; letters indicate significant differences between populations) and mated nest holders (empty boxes); (B) mean brood size (mm2) of mated nest holders; (C) egg density expressed as the number of eggs within 0.5 × 0.5 cm; (D) egg size (mm2). See Table 1 for site abbreviations and Figure 3 for details on box plot graphs.
Mating success
Not all males who had taken up a nest were successful in also receiving eggs within 72 h and a significant difference in the frequency of mated nest holders was detected between populations (log-likelihood test: χ2 = 44.5, df = 4, P < 0.0001, n = 72; Figure 5A, white bars). The number of mated nest holders did not differ significantly from expected numbers at the high salinity site KR (χ2 = 0.04, P = 0.852, n = 9) nor at intermediate sites (VR: χ2 = 0.08, P = 0.775, n = 7; IP: χ2 = 0.009, P = 0.924, n = 3). For the 2 low salinity sites we found opposing results; while there were significantly more mated nest holders than expected at GO (χ2 = 12.3, P = 0.0005, n = 29), there were significantly less mated nest holders than expected at TV (χ2 = 27.7, P < 0.0001, n=1).
Reproductive success
Brood size (representing male reproductive success) was significantly different between populations (Table 2), with males at the intermediate site IP showing significantly higher reproductive success (mean brood size in mm2± SE: 932 ± 120.8) than males of all other populations (Figure 5B). Broods of all other populations did not significantly differ in size (KR: 648 ± 66.9, VR: 597 ± 60.0, GO: 581 ± 32.6; Figure 5B). Egg removal on broods was detected in 3 out of 5 populations. The highest rate of egg removal via filial cannibalism or egg predation was detected at the high salinity site KR where overall 8 out of 9 broods showed signs of egg removal, of which 5 broods were completely consumed after 72 h during the mating assay. At the intermediate site VR 3 out of 7 broods showed signs of egg removal with 1 brood being completely consumed. At the low salinity site GO only 3 out of 29 broods showed mild signs of egg removal. At IP (and TV: n = 1), no egg removal was detected.
Brood characteristics
Egg density
Egg density was significantly different between populations (Table 2 and Figure 5C) with males of the low salinity site GO having significantly fewer eggs within 0.5 × 0.5 cm (mean egg number ± SE = 33.7 ± 1.1) than males of high and intermediate sites (KR: 43.3 ± 3.4, VR: 48.2 ± 1.9, IP: 48.0 ± 3.8). There was no significant difference between the low salinity site TV (41.3 ± 0.9; Figure 5C) and any of the intermediate and high salinity sites.
Egg size
Egg size data suggest males of intermediate salinity sites (VR and IP) to receive the smallest eggs. Egg size differed significantly between all populations, except between the high (KR) and the low (GO) salinity sites, where broods contained the biggest eggs (mean egg size in mm2 ± SE: KR: 0.465 ± 0.01, GO: 0.474 ± 0.02; Table 2 and Figure 5D). At the intermediate sites, VR and IP as well as the low salinity site TV males received significantly smaller eggs (VR: 0.267 ± 0.01, IP: 0.305 ± 0.01, and TV: 0.396 ± 0.02; Figure 5D).
Discussion
We found that geographic variation of environmental conditions influences fundamental aspects of mating success in common gobies, which may directly or indirectly affect the sexual selection regime of common goby populations along the salinity gradient in the Baltic Sea Area. Transect data revealed a cline: quantity and quality of nesting resources (M. arenaria) generally decreased with decreasing salinity from West to East (Figure 3A–D) and so did body size of common gobies (Figure 3G, H).
Common gobies have a resource-based mating system, where the availability of mussel shells is crucial for successful mating and reproduction. Because those mussels are marine species, low salinity waters pose suboptimal habitat conditions for them (Kube et al. 1996). One of only few bivalve species that occurs throughout the full salinity gradient at all 5 sampled sites is the soft-shell clam M. arenaria. At low salinity sites it is also the only one of sufficient size to serve as nest resource for P. microps. As expected, we found a considerable decrease in the density of M. arenaria in salinities of 7 PSU and lower (Figure 3C). Interestingly, it seems that the threshold for growth of M. arenaria is roughly around 15 PSU (according to our measurements between 19 and 13 PSU), because despite its high density at IP (12 PSU), its size was small, comparable to that of the shells measured at the low salinity sites GO (7 PSU) and TV (5 PSU; Figure 3D). Our findings support those from Matthiessen (1960), who showed that M. arenaria can survive in salinities as low as 4 PSU; however, the feeding rate was already negatively affected below 15 PSU, as the pumping rate is then significantly reduced, leading to smaller shell sizes. Therefore, the Baltic Sea Area with its salinity gradient provides a natural experimental setting for the salinity tolerance of soft-shell clams (Strasser 1999). This in turn directly links to a clinal variation in abundance and size of the most-used nesting resources for common gobies.
Differences in body size were already found in a previous study on the closely related sand goby Pomatoschistus minutus, which compared the mode of sexual selection between the high nest availability site (KR) and the low nest availability site (TV; Forsgren et al. 1996a). Males of KR (high salinity) were also found to be larger than males of TV (low salinity); however, no clear conclusion about male size differences was drawn and no link to differences in salinity between high and low nest availability sites were hypothesized as a possible explanation. With our extended study on P. microps, incorporating a total of 5 populations spanning the entire salinity gradient, we fill the gap of knowledge on how body sizes vary between high and low salinity sites. Results show a linear decrease of body size in common gobies along the salinity gradient in the Baltic Sea Area from West (KR) to East (TV; Figure 3G, H). The common goby is a marine species, which originated from the Mediterranean Sea (Simonovic 1999). Thus, low salinity is likely associated with physiological stress. Fish with high metabolic costs of osmoregulation often compensate by allocating less energy to growth with 20% to >50% of the total fish energy budget being dedicated to osmoregulation (reviewed in Boeuf and Payan 2001). This could explain a decrease in body size of common gobies with decreasing salinity and increasing costs for osmoregulation (Boeuf and Payan 2001; Glover et al. 2012; Passow et al. 2015). Therefore, our findings that fish body size as well as shell size (and shell density) decrease with decreasing salinity could be explained by both species originating from a fully marine background being affected in a similar way by low salinity conditions. Interestingly, DeFaveri and Merila (2014) found the opposite pattern for Baltic Sea sticklebacks Gasterosteus aculeatus. Here, juveniles grew to larger sizes in the low salinity treatment in a common garden setup. A potential solution for this discrepancy may be their ability to fully cope with freshwater and an often anadromous lifestyle for brackish populations in 3-spine sticklebacks (McKinnon and Rundle 2002) as well as a trade-off between investment in growth and lateral plating favoring allocation into growth for freshwater populations and into armaments for marine populations (Marchinko and Schluter 2007; Barrett et al. 2009).
Another explanation could be that decreased salinity originally only led to a decrease in shell size, and that this, secondarily, led to the observed habitat-specific size differences in gobies using these shells as nesting resources. It is possible that small common goby males (as well as females) of low salinity habitats facing small nests had over time an evolutionary advantage by actually being able to utilize these smaller clams as nesting resources and hence fit inside small nests. Size-assortative nest choice has been shown for common gobies (Magnhagen and Vestergaard 1993) and many other fish species with a resource-based mating system and paternal care (Kvarnemo 1995; Natsumeda 1998; Takegaki et al. 2008). Even though there is evidence from a variety of fish species, including P. microps (Hastings 1992; Magnhagen and Vestergaard 1993), that larger nests generally contain more eggs resulting in higher reproductive success, there seems to be a trade-off between maximizing surface area for egg deposition and minimizing costs of nest maintenance and defense. However, due to limited clam size and quality, for common goby males in low salinity habitats, a free choice between large and small nests is rare. It is likely that sexual selection is not favoring larger body sizes in low salinity habitats. Indeed, in the low salinity population TV, females have no clear preference for larger males (Heubel KU, submitted for publication). Hence, over time this may lead to local adaptation (Burger and Lynch 1995; Kawecki and Ebert 2004) and accordingly overall smaller common gobies inhabiting low salinity habitats with smaller nesting resources compared with larger males inhabiting high salinity habitats with large nests. The strong positive correlation between male size and clam size (Figure 4) highlights the effect salinity can have on species’ metabolism and therefore growth rate, but could also point toward an interesting link between natural and sexual selection. Future studies should investigate this further, to elucidate which role, if any, sexual selection might play in shaping these patterns.
Why did common gobies colonize the eastern parts of the Baltic Sea Area at all, if adverse conditions caused a decrease in nest quality and quantity, and a reduction in growth rate? One explanation may be that high population densities can result in lower fitness for individuals who settled originally in the best possible habitat (high salinity, western Baltic Sea Area). Thus, if a population density would be reached at which expected fitness in a poorer habitat would be as high as in the best habitat, colonization of the poorer habitat (low salinity, eastern Baltic Sea Area) may begin (Fretwell 1972). Our results on individual counts along transects generally support this theory, suggesting low population densities in low salinity habitats (GO and TV) and the highest population densities at intermediate sites (KE and IP; Figure 3E). According to this scenario, however, one would expect to find the highest population density at the high salinity site (KR), yet numbers of individuals along transects at KR were almost as low as at TV, the site with the lowest salinity. One very likely explanation for this observation are sea level fluctuations of up to 2 m around KR due to deep low pressure passages over the Bothnian Bay, combined with high pressure over the southern Baltic Sea Area (Swedish Meteorological and Hydrological Institute). During the sampling period in KR sea level was below normal (early breeding season 2013/2014: −29/−258 mm, late breeding season 2013: −128 mm), which could have led to common gobies staying in deeper waters rather than start breeding in shallow, unpredictable coastal areas. We, therefore, recommend to treat results on individual densities at the local site KR very cautiously, due to unusual meteorological and hydrological abnormalities during sampling. Alternatively, intermediate salinity levels might constitute optimal habitat conditions due to high nesting resource availability and intermediate abiotic factors indicated by high population density (Gilliers et al. 2006). However, all population density data reported here have to be treated with caution as we only sampled 1 site per population, thus we do not have information on local variation of individual counts within populations.
Furthermore, individual counts along transects differed not only between populations but also from early to late in the breeding season. Generally, more fish were counted early than late in the breeding season. Common gobies are annual fish and it was shown for the closely related sand goby that males facing intra-sexual competition died earlier than males not competing, because of increased stress levels and energy depletion (Lindström 2001). A drop in population size as the breeding season progresses might be caused by a depletion of energy reserves after reproduction leading to high mortality of this annual fish. Similarly, also in the closely related 2-spotted goby Gobiusculus flavescens individual counts along transects dropped toward the end of the breeding season (Forsgren et al. 2004).
The OSR was significantly more male biased at the low salinity site GO and showed a trend for a male bias for the other low salinity site TV as well as the high salinity site KR, while intermediate salinity sites were clearly female biased (Figure 3F). These findings however, coincide with the overall very low numbers of individuals at the low and high salinity sites, potentially making it more likely to count stationary males sitting in their nests (defined as ready-to-mate), than counting free swimming ready-to-mate females. No change in the OSR was found over the breeding season. In contrast, 2-spotted gobies G. flavescens inhabiting the high salinity sites in the Baltic Sea Area showed a shift from male-to-female-biased OSR over the season (Forsgren et al. 2004). Nevertheless, more frequent sampling could detect subtle differences across time of the breeding season and reflect the whole progress of 1 breeding season for the different sites more accurately. On the other hand, such a clear temporal shift in the OSR may not exist in P. microps. Overall, we are cautious with our interpretation of OSR results because of small sample sizes (KR, GO, TV) and therefore larger variation between sites.
Results of the mating assays corresponded well with data on natural nests collected along transects. The nest occupation rate of artificial nests mirrored natural nest shortage; low salinity sites showed the highest artificial nest occupation rate (natural nest availability: low), followed by KR and KE (natural nest availability: intermediate), and IP (natural nest availability: high; Figure 5A). These findings are similar to results on artificial nest occupation rate of a field study on sand gobies in KR and TV where males at TV occupied more nests and did so faster than males at KR (Forsgren et al. 1996a).
The frequencies of the mating success by males occupying an artificial nest were, however, unexpected. While all nest holding males of the low salinity site GO received eggs, this was true for only 1 of the nest holding males at the other low salinity site TV (Figure 5A). A plausible explanation is lacking here, because both GO and TV showed a rather male-biased OSR. However, the conducted mating assay represents a single, short time frame (72 h) only, during which abiotic factors like unstable weather conditions may cause females to reduce spawning (authors personal observation). In fact, during the period the mating assay was carried out in 2012, water temperatures at all sites ranged between 15°C and 20°C, but did not exceed 14°C in TV. The rise of water temperatures during the breeding season of common gobies starts later in the North and East, resulting in spatial–temporal variation in water temperature in the Baltic Sea Area. Water temperature plays a crucial role in female egg development as well as in the duration the eggs need to hatch, and therefore affects the reproductive cycle of both sexes as well as the OSR (Kvarnemo and Ahnesjö 1996; Ahnesjö et al. 2001).
The highest reproductive success (brood size measured as area) was found at the intermediate population IP, which at the same time was also the population with the lowest artificial nest occupation rate despite highest population density. Other studies suggested that females of various fish species with paternal care prefer to lay eggs in nests, which already contain eggs (Jamieson 1995; Forsgren et al. 1996b; Goulet 1998). This might explain why only 60% of nest holding males received eggs at IP, but all of these males were guarding large broods (suggesting clutches of 2 or more females). However, the expression of a preference for nests that already contain eggs may also vary across populations (Andren and Kvarnemo 2014). Females originating from TV showed a preference for empty nests even under experimentally manipulated levels of female mate competition (Heubel KU, submitted for publication). Broods at KR, VR, and GO were similar in size (Figure 5B). By definition, the variation in mating and reproductive success is determining the strength and direction of sexual selection within populations as well as between populations (Howard 1983).
Interestingly, although brood sizes and egg sizes at the high salinity site KR and the low salinity site GO were similar, the egg density was higher at KR than at GO (Figure 5B, d). Broods of intermediate sites (VR and IP) contained the smallest eggs at high density and broods of the low salinity site TV contained eggs of intermediate size and density (Figure 5C, d). A reduced density of eggs in a brood may prevent the spread of Saprolegnia water mould infections which are especially prevalent in low salinity habitats (Vallon et al. 2016b; Vallon and Heubel 2017). In addition, females and nesting resources of low salinity sites are smaller than those of high salinity sites, and body size is directly linked to egg size (Chambers and Leggett 1996) and fecundity in many fish species (Koops et al. 2004). It remains to be studied whether females of low salinity sites may compensate these trade-offs by investing in egg quality (size) rather than in egg quantity, because large eggs imply larger offspring, which increases their survival rate especially during the first critical days when larvae still nourish from their yolk sac (Tamada and Iwata 2005; Allen et al. 2008). Our results on differences between populations in brood size, egg size, and egg density point at thus far underappreciated trade-offs and highlight that inappropriate techniques chosen to estimate reproductive success can lead to false conclusions. Finally, our data on variation of population parameters, mating prospects, and reproductive outcomes along an environmental gradient provide the crucial body of data on ecological properties and its variations as eagerly requested for future studies on sexual selection in natural populations (Gosden and Svensson 2008; Cornwallis and Uller 2010; Miller and Svensson 2014).
In conclusion, our results suggest that low salinity sites in the Baltic Sea Area pose a rather suboptimal habitat choice for common gobies. Mussel shells necessary for reproduction decrease in quality and quantity, and the population density and growth rate of P. microps itself is reduced, suggesting that geographic variation of abiotic and biotic factors can strongly influence populations’ life history and prospects for mating. We demonstrated the importance of considering environmental parameters, such as resource availability among populations, in studies investigating a species’ sexual selection regime. In particular environmental gradients are likely to promote a basis for environmental-context-dependent plasticity of sexual selection. Neglecting differences in abiotic factors between populations may lead to false conclusions about sex role dynamics and the overall outcome of sexual selection. We encourage standardized common garden experiments to empirically test the effect of salinity on physiological traits such as body size and on the strength and direction of sexual selection.
Acknowledgments
We like to thank Hermann Mück, Andreas Svensson, and Emma Tomalty for their help in the field; Nils Anthes, Ralph Dobler, Karen de Jong, and Henri Thomassen for their general advice and comments throughout the project. We thank Ola Svensson for extensive assistance with permits. Helpful suggestions and comments by Rüdiger Riesch and 2 anonymous reviewers greatly improved the manuscript. We thank the Lovén Center for Marine Infrastructure Kristineberg, The Marine Biological Research Center in Kerteminde, the Biological Research Station Ar on Gotland, and Tvärminne Zoological Station for providing research facilities. All applicable international, national, and institutional guidelines for the care and use of animals were followed (permits Dnr.10-2012 and Dnr. 86-2013).
Funding
This project was financially supported by a grant from the Volkswagen foundation (Project 84 846, 92 002 to to K.U.H.) and by the European Community for transnational access (ASSEMBLE [grant agreement no. 227799]).
References
- Ahnesjö I, Kvarnemo C, Merilaita S, 2001. Using potential reproductive rates to predict mating competition among individuals qualified to mate. Behav Ecol 12:397–401. [Google Scholar]
- Allen RM, Buckley YM, Marshall DJ, 2008. Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am Nat 171:225–237. [DOI] [PubMed] [Google Scholar]
- Almada VC, Goncalves EJ, Oliveira RF, Santos AJ, 1995. Courting females: ecological constraints affect sex-roles in a natural population of the Blenniid fish Salaria pavo. Anim Behav 49:1125–1127. [Google Scholar]
- Andersson M, 1994. Sexual Selection. Princeton (NJ): Princeton University Press. [Google Scholar]
- Andren MN, Kvarnemo C, 2014. Filial cannibalism in a nest-guarding fish: females prefer to spawn in nests with few eggs over many. Behav Ecol Sociobiol 68:1565–1576. [Google Scholar]
- Barrett RDH, Rogers SM, Schluter D, 2009. Environment specific pleiotropy facilitates divergence at the ectodysplasin locus in the threespine stickleback. Evolution 63:2831–2837. [DOI] [PubMed] [Google Scholar]
- Bergmann C, 1847. Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien 3:595–708. [Google Scholar]
- Blackburn TM, Gaston KJ, Loder N, 1999. Geographic gradients in body size: a clarification of Bergmann’s rule. Divers Distrib 5:165–174. [Google Scholar]
- Boeuf G, Payan P, 2001. How should salinity influence fish growth? Comp Biochem Physiol C Toxicol Pharmacol 130:411–423. [DOI] [PubMed] [Google Scholar]
- Bonduriansky R, Rowe L, 2003. Interactions among mechanisms of sexual selection on male body size and head shape in a sexually dimorphic fly. Evolution 57:2046–2053. [DOI] [PubMed] [Google Scholar]
- Borg AA, Forsgren E, Magnhagen C, 2002. Plastic sex-roles in the common goby: the effect of nest availability. Oikos 98:105–115. [Google Scholar]
- Brock V, 1980. The geographical distribution of Cerastoderma [cardium] edule (L.) and Cerastoderma lamarcki (Reeve) in the Baltic and adjacent seas related to salinity and salinity fluctuations. Ophelia 19:207–214. [Google Scholar]
- Burger R, Lynch M, 1995. Evolution and extinction in a changing environment: a quantitative-genetic analysis. Evolution 49:151–163. [DOI] [PubMed] [Google Scholar]
- Chambers RC, Leggett WC, 1996. Maternal influences on variation in egg sizes in temperate marine fishes. Am Zool 36:180–196. [Google Scholar]
- Collins MA, Bailey DM, Ruxton GD, Priede IG, 2005. Trends in body size across an environmental gradient: a differential response in scavenging and non-scavenging demersal deep-sea fish. Proc R Soc B 272:2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwallis CK, Uller T, 2010. Towards an evolutionary ecology of sexual traits. Trends Ecol Evol 25:145–152. [DOI] [PubMed] [Google Scholar]
- DeFaveri J, Merila J, 2014. Local adaptation to salinity in the three-spined stickleback?. J Evol Biol 27:290–302. [DOI] [PubMed] [Google Scholar]
- Dubey S, Brown GP, Madsen T, Shine R, 2009. Sexual selection favours large body size in males of a tropical snake (Stegonotus cucullatus, Colubridae). Anim Behav 77:177–182. [Google Scholar]
- Emlen ST, Oring LW, 1977. Ecology, sexual selection, and evolution of mating systems. Science 197:215–223. [DOI] [PubMed] [Google Scholar]
- Evans DH, Claiborne JB, 2008. Osmotic and ionic regulation: cells and animals In: Evans DH, editor. Osmotic and Ionic Regulation in Fishes. Boca Raton: CRC Press, 295–366. [Google Scholar]
- Fonds M, van Buurt G, 1974. The influence of temperature and salinity on the development and survival of goby eggs (Pisces, Gobiidae). Hydrobiol Bull 8:110–116. [Google Scholar]
- Forsgren E, Amundsen T, Borg AA, Bjelvenmark J, 2004. Unusually dynamic sex roles in a fish. Nature 429:551–554. [DOI] [PubMed] [Google Scholar]
- Forsgren E, Karlsson A, Kvarnemo C, 1996b. Female sand gobies gain direct benefits by choosing males with eggs in their nests. Behav Ecol Sociobiol 39:91–96. [Google Scholar]
- Forsgren E, Kvarnemo C, Lindström K, 1996a. Mode of sexual selection determined by resource abundance in two sand goby populations. Evolution 50:646–654. [DOI] [PubMed] [Google Scholar]
- Fretwell SD, 1972. Populations in a Seasonal Environment. Princeton (NJ): Princeton University Press. [PubMed] [Google Scholar]
- Gamble S, Lindholm AK, Endler JA, Brooks R, 2003. Environmental variation and the maintenance of polymorphism: the effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecol Lett 6:463–472. [Google Scholar]
- Gillespie SR, Tudor MS, Moore AJ, Miller CW, 2014. Sexual selection is influenced by both developmental and adult environments. Evolution 68:3421–3432. [DOI] [PubMed] [Google Scholar]
- Gilliers C, Le Pape O, Desaunay Y, Morin J, Guerault D. et al. , 2006. Are growth and density quantitative indicators of essential fish habitat quality? An application to the common sole Solea solea nursery grounds. Estuar Coast Shelf Sci 69:96–106. [Google Scholar]
- Glover DC, DeVries DR, Wright RA, 2012. Effects of temperature, salinity and body size on routine metabolism of coastal largemouth bass Micropterus salmoides. J Fish Biol 81:1463–1478. [DOI] [PubMed] [Google Scholar]
- Gosden TP, Svensson EI, 2008. Spatial and temporal dynamics in a sexual selection mosaic. Evolution 62:845–856. [DOI] [PubMed] [Google Scholar]
- Goulet D, 1998. Spawning success in the damselfish Amblyglyphidodon leucogaster: the influence of eggs in the nest. Anim Behav 55:651–664. [DOI] [PubMed] [Google Scholar]
- Gwynne DT, Simmons LW, 1990. Experimental reversal of courtship roles in an insect. Nature 346:172–174. [Google Scholar]
- Hargreaves AL, Eckert CG, 2014. Evolution of dispersal and mating systems along geographic gradients: implications for shifting ranges. Funct Ecol 28:5–21. [Google Scholar]
- Hastings PA, 1992. Nest-site size as a short-term constraint on the reproductive success of paternal fishes. Environ Biol Fish 34:213–218. [Google Scholar]
- HELCOM, 1992. Convention on the Protection of the Marine Environment of the Baltic Sea Area, 1992.
- HELCOM, 1993. First Assessment of the State of the Coastal Waters of the Baltic Sea. Balt Sea Environ Proc 54.
- HELCOM, 1996. Third periodic assessement of the state of the marine envrionment of the Baltic Sea, 1989–1993. Executive summary. Balt Sea Environ Proc 64A.
- Hill GE, 1994. Geographic variation in male ornamentation and female mate preference in the house finch: a comparative test of models of sexual selection. Behav Ecol 5:64–73. [Google Scholar]
- Howard RD, 1983. Sexual selection and variation in reproductive success in a long-lived organism. Am Nat 122:301–325. [Google Scholar]
- Jamieson I, 1995. Female fish prefer to spawn in nests with eggs for reasons of mate choice copying or egg survival. Am Nat 145:824–832. [Google Scholar]
- Janicke T, David P, Chapuis E, 2015. Environment-dependent sexual selection: Bateman’s parameters under varying levels of food availability. Am Nat 185:756–768. [DOI] [PubMed] [Google Scholar]
- Jansen JM, Koutstaal A, Bonga SW, Hummel H, 2009. Salinity-related growth rates in populations of the European clam Macoma balthica and in field transplant experiments along the Baltic Sea salinity gradient. Mar Freshw Behav Physiol 42:157–166. [Google Scholar]
- Jennions MD, Kokko H, 2010. Sexual selection In: Westneat DF, Fox CW, editors. Evolutionary Behavioral Ecology. Oxford: Oxford University Press, 343–364. [Google Scholar]
- Kawecki TJ, Ebert D, 2004. Conceptual issues in local adaptation. Ecol Lett 7:1225–1241. [Google Scholar]
- Kim S-Y, Metcalfe NB, da Silva A, Velando A, 2017. Thermal conditions during early life influence seasonal maternal strategies in the three-spined stickleback. BMC Ecol 17:34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Jennions MD, 2008. Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948. [DOI] [PubMed] [Google Scholar]
- Kokko H, Klug HM, Jennions MD, 2012. Unifying cornerstones of sexual selection: operational sex ratio, Bateman gradient, and the scope for competitive investment. Ecol Lett 15:1340–1351. [DOI] [PubMed] [Google Scholar]
- Kokko H, Rankin DJ, 2006. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos Trans R Soc Lond B 361:319–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops MA, Hutchings JA, McIntyre TM, 2004. Testing hypotheses about fecundity, body size and maternal condition in fishes. Fish Fish 5:120–130. [Google Scholar]
- Kube J, Peters C, Powilleit M, 1996. Spatial variation in growth of Macoma balthica and Mya arenaria (Mollusca, Bivalvia) in relation to environmental gradients in the Pomeranian Bay (Southern Baltic Sea). Arch Fish Mar Res 44:81–93. [Google Scholar]
- Kvarnemo C, 1995. Size-assortative nest choice in the absence of competition in males of the sand goby Pomatoschistus minutus. Environ Biol Fish 43:233–239. [Google Scholar]
- Kvarnemo C, Ahnesjö I, 1996. The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski MA, Sullivan BK, 2002. Geographic variation in sexual selection among populations of an iguanid lizard Sauromalus obesus (=ater). Evolution 56:2039–2051. [DOI] [PubMed] [Google Scholar]
- Liao WB, Lu X, 2011. Variation in body size, age and growth in the Omei treefrog (Rhacophorus omeimontis) along an altitudinal gradient in western China. Ethol Ecol Evol 23:248–261. [Google Scholar]
- Lindsey CC, 1966. Body sizes of poikilotherm vertebrates at different latitudes. Evolution 20:456–465. [DOI] [PubMed] [Google Scholar]
- Lindström K, 1988. Male–male competition for nest sites in the sand goby Pomatoschistus minutus. Oikos 53:67–73. [Google Scholar]
- Lindström K, 2001. Effects of resource distribution on sexual selection and the cost of reproduction in sand gobies. Am Nat 158:64–74. [DOI] [PubMed] [Google Scholar]
- Lindström K, Pampoulie C, 2005. Effects of resource holding potential and resource value on tenure at nest sites in sand gobies. Behav Ecol 16:70–74. [Google Scholar]
- Magnhagen C, Vestergaard K, 1993. Brood size and offspring age affect risk-taking and aggression in nest-guarding common gobies. Behaviour 125:233–243. [Google Scholar]
- Marchinko KB, Schluter D, 2007. Parallel evolution by correlated response: lateral plate reduction in threespine stickleback. Evolution 61:1084–1090. [DOI] [PubMed] [Google Scholar]
- Matthiessen GC, 1960. Observations on the ecology of the soft clam Mya arenaria in a salt pond. Limnol Oceanogr 5:291–300. [Google Scholar]
- McKinnon JS, Rundle HD, 2002. Speciation in nature: the three-spine stickleback model systems. Trends Ecol Evol 17:480–488. [Google Scholar]
- Miller CW, Svensson EI, 2014. Sexual selection in complex environments. Ann Rev Entomol 59:427–445. [DOI] [PubMed] [Google Scholar]
- Miller PJ, 1975. Age–structure and life-span in common goby Pomatoschistus microps. J Zool 177:425–448. [Google Scholar]
- Monteiro N, Cunha M, Ferreira L, Vieira N, Antunes A. et al. , 2017. Parabolic variation in sexual selection intensity across the range of a cold-water pipefish: implications for susceptibility to climate change. Glob Change Biol 23:3600–3609. [DOI] [PubMed] [Google Scholar]
- Monteiro NM, Lyons DO, 2012. Stronger sexual selection in warmer waters: the case of a sex role reversed pipefish. PLoS ONE 7:e44251.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EC, 1985. Bergmanns rule, seasonality, and geographic variation in body size of house sparrows. Evolution 39:1327–1334. [DOI] [PubMed] [Google Scholar]
- Natsumeda T, 1998. Size-assortative nest choice by the Japanese fluvial sculpin in the presence of male–male competition. J Fish Biol 53:33–38. [Google Scholar]
- Nyman K-J, 1953. Observations on the behaviour of Gobius microps. Acta Soc Fauna Flora Fenn 69:1–11. [Google Scholar]
- Ojaveer H, Jaanus A, MacKenzie BR, Martin G, Olenin S. et al. , 2010. Status of biodiversity in the Baltic Sea. PLoS ONE 5:e12467.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA, Sutherland WJ, 1986. Ideal free distributions when individuals differ in competitive ability: phenotype-limited ideal free models. Anim Behav 34:1222–1242. [Google Scholar]
- Passow CN, Greenway R, Arias-Rodriguez L, Jeyasingh PD, Tobler M, 2015. Reduction of energetic demands through modification of body size and routine metabolic rates in extremophile fish. Physiol Biochem Zool 88:371–383. [DOI] [PubMed] [Google Scholar]
- Peck MA, Baumann H, Bernreuther M, Clemmesen C, Herrmann JP. et al. , 2012. The ecophysiology of Sprattus sprattus in the Baltic and North seas. Progr Oceanogr 103:42–57. [Google Scholar]
- Core Team R, 2012. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rundle HD, Chenoweth SF, Blows MW, 2006. The roles of natural and sexual selection during adaptation to a novel environment. Evolution 60:2218–2225. [PubMed] [Google Scholar]
- Rypel AL, 2014. The cold-water connection: Bergmann’s rule in North American freshwater fishes. Am Nat 183:147–156. [DOI] [PubMed] [Google Scholar]
- Siepielski AM, DiBattista JD, Carlson SM, 2009. It’s about time: the temporal dynamics of phenotypic selection in the wild. Ecol Lett 12:1261–1276. [DOI] [PubMed] [Google Scholar]
- Simonovic PD, 1999. Phylogenetic relationship of Ponto-Caspian gobies and their relationship to Atlantic-Mediterranean Gobiinae. J Fish Biol 54:533–555. [Google Scholar]
- Smith KF, Brown JH, 2002. Patterns of diversity, depth range and body size among pelagic fishes along a gradient of depth. Glob Ecol Biogeogr 11:313–322. [Google Scholar]
- St Mary CM, Gordon E, Hale RE, 2004. Environmental effects on egg development and hatching success in Jordanella floridae, a species with parental care. J Fish Biol 65:760–768. [Google Scholar]
- Stillwell RC, Morse GE, Fox CW, 2007. Geographic variation in body size and sexual size dimorphism of a seed-feeding beetle. Am Nat 170:358–369. [DOI] [PubMed] [Google Scholar]
- Strasser M, 1999. Mya arenaria: an ancient invader of the North sea coast. Helgolander Meeresunters 52:309–324. [Google Scholar]
- Takegaki T, Matsumoto Y, Tawa A, Miyano T, Natsukari Y, 2008. Size-assortative nest preference in a paternal brooding blenny Rhabdoblennius ellipes (Jordan & Starks). J Fish Biol 72:93–102. [Google Scholar]
- Tamada K, Iwata K, 2005. Intra-specific variations of egg size, clutch size and larval survival related to maternal size in amphidromous Rhinogobius goby. Environ Biol Fish 73:379–389. [Google Scholar]
- Vallon M, Anthes N, Heubel KU, 2016a. Water mold infection but not paternity induces selective filial cannibalism in a goby. Ecol Evol 6:7221–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon M, Grom C, Kalb N, Sprenger D, Anthes N. et al. , 2016b. You eat what you are: personality-dependent filial cannibalism in a fish with paternal care. Ecol Evol 6:1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon M, Heubel KU, 2016. Old but gold: males preferentially cannibalize young eggs. Behav Ecol Sociobiol 70:569–573. [Google Scholar]
- Vallon M, Heubel KU, 2017. Egg density and salinity influence filial cannibalism in common gobies. Behav Ecol Sociobiol 71:159. [Google Scholar]
- Wacker S, Amundsen T, 2014. Mate competition and resource competition are inter-related in sexual selection. J Evol Biol 27:466–477. [DOI] [PubMed] [Google Scholar]
- Wacker S, Amundsen T, Forsgren E, Mobley KB, 2014. Within-season variation in sexual selection in a fish with dynamic sex roles. Mol Ecol 23:3587–3599. [DOI] [PubMed] [Google Scholar]
- Westerbom M, Kilpi M, Mustonen O, 2002. Blue mussels Mytilus edulis at the edge of the range: population structure, growth and biomass along a salinity gradient in the north-eastern Baltic Sea. Mar Biol 140:991–999. [Google Scholar]
- Zar JH, 1984. Biostatistical Analysis. Princeton (NJ): Prentice-Hall. [Google Scholar]