Abstract
Background
The purpose of this study was to examine selected measures of racial and ethnic disparities in the reported incidence of syphilis and gonorrhea from 1981 to 2013 in the United States.
Methods
For each year from 1981 to 2013, we calculated values for five disparity measures (Gini coefficient, two versions of the index of disparity, population attributable proportion, and the black-to-white rate ratio) for five racial/ethnic categories (Non-Hispanic White, Non-Hispanic Black, Hispanic, American Indian/Alaska Native, and Asian/Pacific Islander). We also examined annual and 5-year changes to see if the disparity measures agreed on the direction of change in disparity.
Results
With a few exceptions, the disparity measures increased from 1981 to 1993 and decreased from 1993 to 2013, whereas syphilis and gonorrhea rates decreased for most groups from 1981 to 1993 and increased from 1993 to 2013. Overall, the disparity measures we examined were highly correlated with one another, particularly when examining 5-year changes rather than annual changes in disparity. For example, all five measures agreed on the direction of change in the disparity of syphilis in 56% of the annual comparisons and in 82% of the 5-year comparisons.
Conclusions
Although the disparity measures we examined were generally consistent with one another, these measures can sometimes yield divergent assessments of whether racial/ethnic disparities are increasing or decreasing for a given STD from one point in time to another, as well as divergent assessments of the relative magnitude of the change.
Racial and ethnic disparities in sexually transmitted disease (STD) rates have been documented extensively,1–4 and a number of summary measures can be used to quantify these disparities.2,5–9 For example, the black-to-white rate ratio (e.g., the gonorrhea rate per 100,000 population for blacks divided by that of whites) is commonly used in national STD surveillance reports to illustrate racial/ethnic disparities in reported STD rates.10 Although assessments of racial/ethnic disparities in STDs are common, to our knowledge no study has examined trends in STD disparities over time using several different measures of disparity. Such assessments are useful not only to illustrate changes in disparity over time, but also to inform the potential usefulness of various disparity metrics for purposes such as measuring changes in disparity or as performance indicators for STD prevention activities designed to reduce disparities.
Hoover and colleagues (2008) provide examples of methods for measuring disparities in STD rates and calculating changes in these measures over time.9 Their illustration focused on three main disparity measures: absolute disparities in rates (between the group of interest vs. the reference group), relative disparities in rates, and the index of disparity for use when comparing more than two groups in a population.9 We expand the work by Hoover and colleagues by calculating values for a wider range of disparity measures and by analyzing and comparing changes in these measures over time.
METHODS
Overview
Our general approach was to calculate values for five disparity measures for primary and secondary (P&S) syphilis and gonorrhea from 1981 to 2013 in the United States. We did not include chlamydia in our analysis because of the lack of surveillance data in the early 1980’s. The five disparity measures we examined were the Gini coefficient, two versions of the index of disparity (unweighted and weighted by population subgroup size), the population attributable proportion, and the black-to-white rate ratio, as described in more detail below. We also examined and compared changes in these disparity measures over time. Specifically, we examined whether or not the five measures agreed as to whether racial and ethnic disparity was increasing or decreasing over time, and whether changes in these disparity measures were correlated with one another.
Data
We obtained reported national case numbers for P&S syphilis and gonorrhea for 1981 to 2013 from the National Notifiable Disease Surveillance System (NNDSS). These data are described in more detail in annual STD surveillance reports.10 In our main analyses, we excluded cases where race/ethnicity was not specified as well as cases in which race/ethnicity was reported as “other”. We used population data from the Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics (NCHS) to calculate rates of reported cases of gonorrhea and P&S syphilis. The five racial/ethnic categories we used were: American Indian or Alaska Native; Asian or Pacific Islander; Black, not of Hispanic origin; Hispanic; and White, not of Hispanic origin. In order to examine changes in disparities in gonorrhea and syphilis across the same five race/ethnicity groups from 1981 to 2013, we used NCHS bridged-race population data for 2000–2012. These population estimates bridge the race categories listed in the 1997 Office of Management and Budget (OMB) standards to the five race/ethnicity groups specified in the previous 1977 OMB standards. For example, the 1997 categories of “Asian” and “Native Hawaiian/Other Pacific Islander” were combined into the pre-1997 category of “Asian/Pacific Islander.” More details are provided elsewhere.10 Cases reported in 1997 OMB-compliant categories were able to select more than one race and are mapped to “other” in the bridged data. Thus, it is likely that cases listed as “other” increased over time as more states became OMB-compliant in their reporting. Although we excluded cases listed as “other” in our main analyses, we performed additional analyses to examine the potential influence of omitting these cases, as described later.
Disparity measures
We did not include all known measures of disparity in this analysis. Instead, in order to provide an illustration of changes in disparity measures over time, we selected five commonly used measures of disparity. We included the Gini coefficient because it is a well-known measure in economics and has increasingly been applied in the STD literature.11–19 We included the index of disparity because it is a well-known summary measure of the differences in disease rates across racial and ethnic groups 5,9 and because this index has been used by CDC to examine changes in racial/ethnic health disparities over time.20 We included the population attributable proportion because it is a useful concept with a long history in epidemiology.21,22 Finally, we included the black-white ratio because this measure is commonly used in STD surveillance reports to quantify racial/ethnic disparities in reported STD rates.10 Although the surveillance report provides rate ratios for several different racial/ethnic groups (vs. non-Hispanic whites), we included only the black-to-white ratio for comparative purposes, to illustrate how the rate ratio for two racial/ethnic groups compares to summary measures of disparity across all racial/ethnic groups.
Gini coefficient
The Gini coefficient is commonly used to quantify inequalities in income distribution.12 However, the Gini coefficient has been adapted and applied in the STD prevention literature for a range of purposes, such as quantifying the distribution of STDs across census tracts, counties, states, and other geographic units,13–16 assessing the geographic distribution of sex workers,17 examining the distribution of clients across sex workers,18 and to illustrate the concentration of sex acts and sex partnerships within the most active members of the population.19
To calculate the Gini coefficient for a given STD in a given year, the racial/ethnic groups were ranked from 1 to 5 according to the STD rate (i = 1 denotes the group with the lowest STD rate and i = 5 denotes the group with the highest STD rate in the given year). The Gini coefficient (G) was calculated as:
where Yi is the cumulative percentage of cases of the given STD occurring in Group 1 through Group i, Xi is the cumulative percentage of the population accounted for by Group 1 though Group i, and X0 and Y0 are both 0.15 The Gini coefficient ranges from 0 (no disparity) to 1 (maximum disparity).
Index of disparity (ID)
The index of disparity (ID) was presented by Pearcy and Keppel (2002) as a summary measure of disease incidence across population groups, where the population groups could be defined by race/ethnicity, educational status, income level, or other relevant factor.6 For the purposes of this study, we calculated the ID based on race/ethnicity for a given STD in a given year, as follows:
where R is the overall rate of the given STD, and ri is the rate of the given STD in racial/ethnic group i.6 Pearcy and Keppel defined the ID as “the average of the absolute differences between rates for specific groups within a population and the overall population rate, divided by the rate for the overall population and expressed as a percentage.”6
We also calculated a weighted version of the ID in which we replaced the average of the absolute differences between ri and R with a weighted average of these absolute differences, where the difference for each group i was weighted by the group’s share of the overall population.
Population attributable proportion
The population attributable proportion (PAP) as a disparity index for a given STD can be described as the proportional decrease in the given STD that would be achieved if all racial/ethnic groups had the same rate of the given STD as the group with the lowest rate of that STD.7,23 Regidor (2004) provided an example of using the PAP to assess mortality inequalities by educational level.7 We calculated the PAP as follows:
where Ci is the number of cases of the given STD in Group i, Ĉi is the number of cases that there would have been in Group i if the rate of the given STD in Group i was that of the group with the lowest rate of that STD, and C is the total number of cases across all five groups.
Black-to-white rate ratio
The black-to-white rate ratio was calculated as Rb/Rw, where Rb is the rate of the given STD for non-Hispanic blacks and Rw is the rate for non-Hispanic whites.9
Correlation of annual changes and 5-year changes in disparity measures
We also examined changes in the disparity measures over 1-year and 5-year intervals. Specifically, for each STD, the annual percentage change in the disparity measure in year t was calculated as (Vt − Vt−1)/Vt−1, where Vt is the value of the given disparity measure in year t. Similarly, the 5-year change in the disparity measure in year t was calculated as (Vt − Vt−5)/Vt−5, where Vt is the value of the given disparity measure in year t. We assessed the correlation between changes in the disparity measures using Pearson correlation coefficients (and p-values).
Agreement across disparity measures regarding direction of change of disparity
As another examination of annual changes in the disparity measures, we assessed whether each disparity measure increased or decreased from the previous year, for both syphilis and gonorrhea. We then examined whether or not the five measures yielded the same assessment of whether racial and ethnic disparity was increasing or decreasing from one year to the next for the given STD. Finally, we repeated this assessment of agreement across disparity measures over 5-year intervals rather than 1-year intervals.
Sensitivity analyses
We repeated the analysis five times, each time examining an extreme scenario in which all STD cases in the race/ethnicity category “other” or “unknown” were assumed to occur in exactly one of the five racial/ethnic groups. For example, we repeated the analysis after assigning all cases in the category “other” or “unknown” to the category “Non-Hispanic White.” Additional sensitivity analyses are described and reported in the supplemental appendix.
RESULTS
Measures of racial/ethnic disparities in STDs
Rates of reported cases of gonorrhea and P&S syphilis by racial/ethnic groups are shown in Figure 1 (panels A and B), and values of the five selected disparity measures are shown in Figure 2 (panels A and B). These results are summarized in Table 1, which includes STD rates and disparity measure values for the first year (1981), an intermediate year (1993), and the final year (2013).
Figure 1.
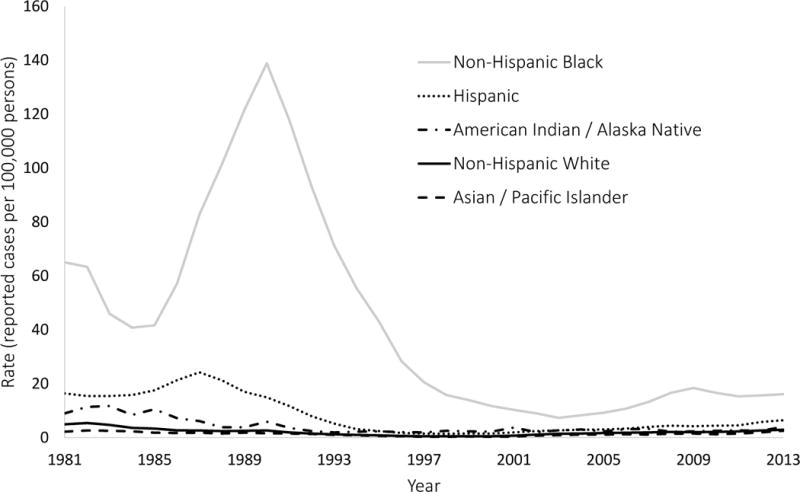
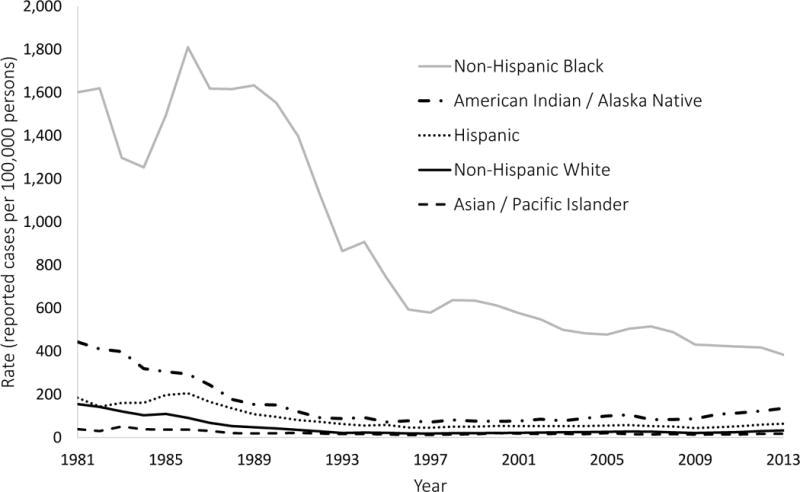
(A & B). Reported rates of syphilis and gonorrhea were obtained from surveillance records maintained by the Centers for Disease Control and Prevention. The rates in this figure exclude cases with missing or unspecified race/ethnicity information.
Figure 2.
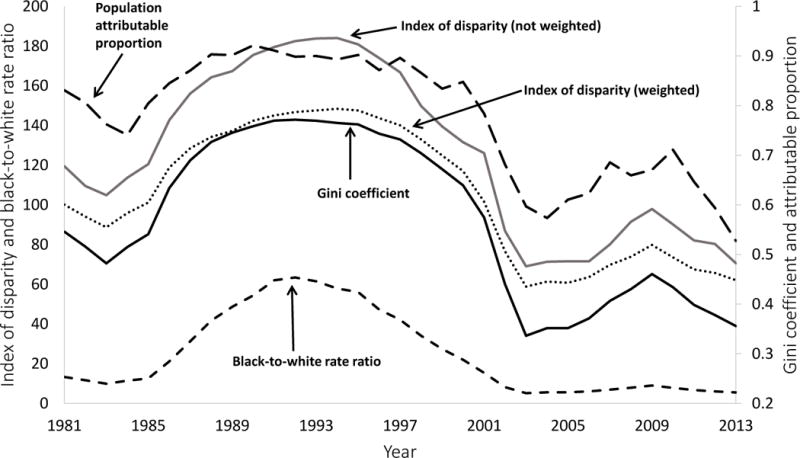
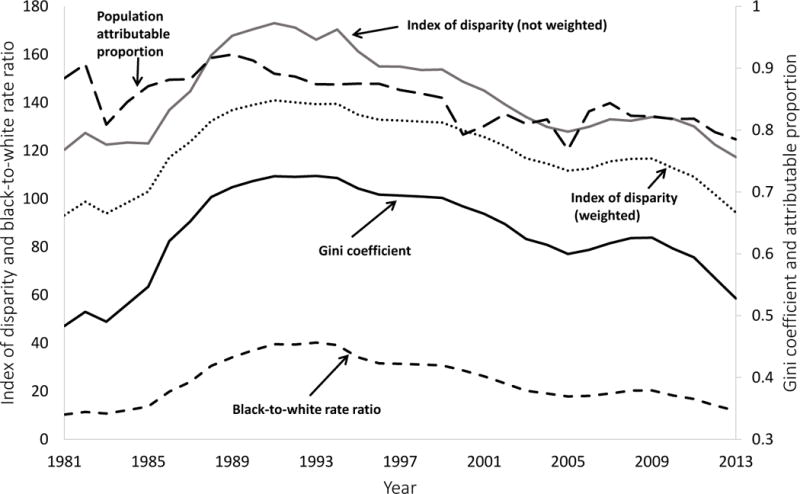
(A & B). The Index of disparity (weighted and not weighted) and the black-to-white rate ratio are shown on the left axis. The population attributable proportion and the Gini coefficient are shown on the right axis. See text for a description of these five measures of disparity.
Table 1.
Syphilis and gonorrhea rates (reported cases per 100,000) by race/ethnicity and values of five measures of racial/ethnic disparities in syphilis and gonorrhea rates in the United States for three selected years: 1981, 1993, and 2013
| Item estimated | Value of measure in 1981 | Value of measure in 1993 | Value of measure in 2013 | Change in measure (%), 1981 to 1993 | Change in measure (%), 1993 to 2013 | Change in measure (%), 1981 to 2013 |
|---|---|---|---|---|---|---|
| Syphilis rates | ||||||
| Non-Hispanic White | 4.9 | 1.2 | 2.9 | −75.5% | 141.7% | −40.8% |
| Non-Hispanic Black | 65.0 | 71.2 | 16.1 | 9.5% | −77.4% | −75.2% |
| Hispanic | 16.4 | 5.2 | 6.4 | −68.3% | 23.1% | −61.0% |
| Asian/Pacific Islander | 2.2 | 1.0 | 2.5 | −54.5% | 150.0% | 13.6% |
| American Indian/Alaska Native | 9.0 | 1.9 | 4.0 | −78.9% | 110.5% | −55.6% |
| Syphilis disparity measures | ||||||
| Gini coefficient | 0.546 | 0.770 | 0.356 | 41.0% | −53.8% | −34.8% |
| Index of disparity, not weighted | 119.5 | 183.8 | 70.6 | 53.9% | −61.6% | −40.9% |
| Index of disparity, weighted | 100.1 | 147.5 | 62.1 | 47.3% | −57.9% | −38.0% |
| Black-to-white rate ratio | 13.3 | 61.5 | 5.5 | 362.2% | −91.0% | −58.5% |
| Population attributable proportion | 0.831 | 0.900 | 0.528 | 8.3% | −41.4% | −36.5% |
| Gonorrhea rates | ||||||
| Non-Hispanic White | 155.4 | 21.5 | 32.4 | −86.2% | 50.7% | −79.2% |
| Non-Hispanic Black | 1,601.4 | 865.0 | 384.5 | −46.0% | −55.5% | −76.0% |
| Hispanic | 184.4 | 62.7 | 64.1 | −66.0% | 2.2% | −65.2% |
| Asian/Pacific Islander | 38.6 | 16.1 | 17.9 | −58.3% | 11.2% | −53.6% |
| American Indian/Alaska Native | 443.6 | 87.5 | 135.5 | −80.3% | 54.9% | −69.5% |
| Gonorrhea disparity measures | ||||||
| Gini coefficient | 0.483 | 0.726 | 0.528 | 50.1% | −27.3% | 9.2% |
| Index of disparity, not weighted | 120.4 | 166.1 | 117.3 | 38.0% | −29.4% | −2.5% |
| Index of disparity, weighted | 93.2 | 139.3 | 94.3 | 49.6% | −32.3% | 1.3% |
| Black-to-white rate ratio | 10.3 | 40.2 | 11.9 | 290.3% | −70.5% | 15.0% |
| Population attributable proportion | 0.884 | 0.874 | 0.785 | −1.1% | −10.2% | −11.2% |
The rates in this table exclude cases where race/ethnicity was not specified. The Black-to-white ratio was calculated based on rates in non-Hispanic blacks and non-Hispanic whites.
The percentage change was calculated using the value of the earlier year in the denominator. For example the change from 1981 to 2013 was calculated as (V2013 − V1981)/V1981, where V2013 is the value of the given measure in 2013 and V1981 is the value of the given measure in 1981.
For all groups except non-Hispanic blacks, syphilis rates decreased between 1981 and 1993 and increased between 1993 and 2013, although these trends were not linear. According to all five measures, disparities in syphilis increased between 1981 and 1993 and decreased between 1993 and 2013, although these trends were also not linear. Incidence rates and disparity measures for gonorrhea followed the same general pattern as syphilis. However, the trends in disparity for gonorrhea were not consistent across all five measures, as the population attributable proportion suggested an overall decrease in disparity from 1981 to 1993, while the other four measures showed an overall increase in disparity over the same time period. Similarly, the population attributable proportion suggested an overall decrease in racial disparity in gonorrhea from 1981 to 2013, while all of the other measures (except for the index of disparity, not weighted) showed an overall increase in disparity over the same time period.
Correlation of annual and 5-year changes in disparity measures
Annual changes in the disparity measures were highly correlated, particularly for syphilis (Table 2). The annual change in each disparity measure was highly correlated with the annual change in each of the other disparity measures, with one borderline exception. For gonorrhea, the correlation coefficient for changes in the population attributable proportion and changes in the black-to-white ratio was 0.349 (p=0.0506). For both syphilis and gonorrhea, the 5-year change in each disparity measure was highly correlated with the 5-year change in each of the other disparity measures (p< 0.0001 for all comparisons).
Table 2.
Correlation between changes in racial disparity measures, 1981 – 2013: Pearson correlation coefficient (p-value)
| Disparity measure | Gini coefficient | Index of disparity, not weighted | Index of disparity, weighted | Black-to-white rate ratio |
|---|---|---|---|---|
| Panel A: Annual changes in syphilis | ||||
|
| ||||
| Index of disparity, not weighted | 0.942 (< 0.0001) | 1 | ||
| Index of disparity, weighted | 0.992 (< 0.0001) | 0.946 (< 0.0001) | 1 | |
| Black-to-white rate ratio | 0.903 (< 0.0001) | 0.865 (< 0.0001) | 0.904 (< 0.0001) | 1 |
| Population attributable proportion | 0.726 (< 0.0001) | 0.665 (< 0.0001) | 0.689 (< 0.0001) | 0.601 (0.0003) |
|
| ||||
| Panel B: 5-year changes in syphilis | ||||
|
| ||||
| Index of disparity, not weighted | 0.989 (< 0.0001) | 1 | ||
| Index of disparity, weighted | 0.996 (< 0.0001) | 0.991 (< 0.0001) | 1 | |
| Black-to-white rate ratio | 0.861 (< 0.0001) | 0.859 (< 0.0001) | 0.854 (< 0.0001) | 1 |
| Population attributable proportion | 0.963 (< 0.0001) | 0.937 (< 0.0001) | 0.949 (< 0.0001) | 0.770 (<0.0001) |
|
| ||||
| Panel C: Annual changes in gonorrhea | ||||
|
| ||||
| Index of disparity, not weighted | 0.858 (< 0.0001) | 1 | ||
| Index of disparity, weighted | 0.990 (< 0.0001) | 0.899 (< 0.0001) | 1 | |
| Black-to-white rate ratio | 0.971 (< 0.0001) | 0.905 (< 0.0001) | 0.968 (< 0.0001) | 1 |
| Population attributable proportion | 0.459 (0.0083) | 0.405 (0.0216) | 0.471 (0.0065) | 0.349 (0.0506) |
|
| ||||
| Panel D: 5-year changes in gonorrhea | ||||
|
| ||||
| Index of disparity, not weighted | 0.933 (< 0.0001) | 1 | ||
| Index of disparity, weighted | 0.995 (< 0.0001) | 0.957 (< 0.0001) | 1 | |
| Black-to-white rate ratio | 0.982 (< 0.0001) | 0.970 (< 0.0001) | 0.989 (< 0.0001) | 1 |
| Population attributable proportion | 0.663 (0.0001) | 0.689 (<0.0001) | 0.676 (<0.0001) | 0.678 (<0.0001) |
This table shows the correlation between annual percentage changes in five measures of racial disparity (Gini coefficient, Index of disparity-weighted and unweighted, black-to-white rate ratio, and the population attributable proportion) in syphilis and gonorrhea from 1981 to 2013. For example, for syphilis, the annual change in the Gini coefficient and the annual change in the Index of disparity (not weighted) were highly correlated, with a Pearson correlation coefficient of 0.942 (p < 0.0001). This analysis included 32 annual percentage changes and 28 5-year changes.
The percentage change was calculated using the value of the earlier year in the denominator. For example, for 1986, the annual percentage change was calculated as (V1986 − V1985)/V1985 and the 5-year percentage change was calculated as (V1986 − V1981)/V1981.
Agreement across disparity measures regarding direction of change of disparity
For syphilis, we examined 32 annual changes in the disparity measures (Table 3, Panel A). In 18 (56.3%) of the 32 instances, all five measures agreed on the direction of change in disparity. In 11 (34.4%) of the 32 years, exactly four measures agreed on the direction of change in disparity. In 3 (9.4%) of the 32 years, exactly three measures agreed on the direction of change in disparity. These results were similar for gonorrhea.
Table 3.
Summary of agreement between the five disparity measures in assessment of annual changes in disparity and 5-year changes in disparity, 1981 – 2013
| Panel A: Annual changes in disparity | ||
|---|---|---|
|
| ||
| Item estimated | Syphilis | Gonorrhea |
| Number of years in which annual change in disparity was assessed | 32 | 32 |
| Number (%) of years in which all five disparity measures agreed on direction of change in disparity | 18 (56.3%) | 19 (59.4%) |
| Number (%) of years in which exactly four measures agreed on direction of change in disparity | 11 (34.4%) | 10 (31.3%) |
| Number (%) of years in which exactly three measures agreed on direction of change in disparity | 3 (9.4%) | 3 (9.4%) |
|
| ||
| Panel B: 5-year changes in disparity | ||
|
| ||
| Item estimated | Syphilis | Gonorrhea |
|
| ||
| Number of years in which 5-year change in disparity was assessed | 28 | 28 |
| Number (%) of years in which all five disparity measures agreed on direction of change in disparity | 23 (82.1%) | 20 (71.4%) |
| Number (%) of years in which exactly four measures agreed on direction of change in disparity | 5 (17.9%) | 7 (25.0%) |
| Number (%) of years in which exactly three measures agreed on direction of change in disparity | 0 (0%) | 1 (3.6%) |
If all five disparity measures increased, or if all five disparity measures decreased in the given time interval, then all five measures agreed on the direction of change in disparity. If exactly four disparity measures increased (and one measure decreased), or if exactly four disparity measures decreased (and one measure increased) in the given time interval, then exactly four measures agreed on the direction of change in disparity. If exactly three disparity measures increased (and two measures decreased), or if exactly three disparity measures decreased (and two measures increased) in the given time interval, then exactly three measures agreed on the direction of change in disparity. Because all of the measures either increased or decreased over the time intervals we examined, at least three of five measures had to agree on the direction of change. The supplemental appendix contains sensitivity analyses in which we limit the analysis to changes of a minimum threshold (i.e., 1%, 3%, and 5%).
The degree of agreement across disparity measures increased when we assessed direction of change in disparity over 5-year intervals rather than annually (Table 3, Panel B). All 5 disparity measures agreed in the majority of instances, and at least 4 of 5 measures agreed in all but one instance.
Cases in which race/ethnicity was listed as “other” or “unknown”
The percentage of reported syphilis cases in the race/ethnic category of “other” in the bridged data increased from 0% in 1981 to 1.0% in 2007 to 1.7% in 2013. The percentage of reported gonorrhea cases in the race/ethnic category of “other” in the bridged data increased from 0% in 1981 to 1.3% in 2007 to 1.5% in 2013.
The percentage of reported syphilis cases with missing (“unknown”) race/ethnicity data in any given year ranged from 1.4% to 19.5%. With the exception of the three-year period from 1983 to 1985, the percentage of reported syphilis cases with missing race/ethnicity data did not exceed 8.4% in any given year and averaged 4.8% across all years. The percentage of reported gonorrhea cases with missing race/ethnicity data in any given year ranged from 16.4% to 25.4% and averaged 21.1% across all years.
Our results did vary in sensitivity analyses when we assigned all of the “other” cases and cases with missing race/ethnicity data to one of the five racial/ethnic groups, particularly when assigning the cases to the smaller groups such as Asian/Pacific Islander and American Indian/Alaska Native. For example, for syphilis, the number of instances in which all five measures agreed on the direction of the annual change in disparity (18 in the base case) was 19, 17, 17, 24, and 7 when all of the “other” and “missing cases” were assigned to non-Hispanic whites, non-Hispanic blacks, Hispanics, Asian/Pacific Islanders, and American Indian/Alaska Natives, respectively (see supplemental appendix for complete results).
DISCUSSION
The disparity measures we examined were quite consistent with one another. The percentage change in any given disparity measure from one year to the next was positively correlated with the percentage change in the other disparity measures. However, from any given year to the next, the various disparity measures could yield divergent results in terms of whether racial/ethnic disparities in STDs are increasing or decreasing as well as in terms of the relative magnitude of the change.
The Gini coefficient and the weighted index of disparity were the most consistently correlated measures, probably due to their similarities in structure in which the population size of the population subgroups are taken into account. The population attributable proportion (PAP) typically had lower correlation coefficients than the other measures when assessing annual changes, probably because the PAP is sensitive to the number of excess cases due to disparity but not how these excess cases are distributed across population subgroups. However, when assessing 5-year changes, the correlation coefficient for the PAP was more consistent with the other measures. The correlation coefficient for the black-to-white rate ratio was often lower than for the other measures, which is not surprising given that the black-to-white ratio reflects disparity in just two groups whereas the other measures are composite measures which account for all five racial/ethnic groups.
The epidemiology of syphilis has changed dramatically over the years we examined, from an infection associated with drug use among heterosexuals to one that is now more associated with MSM and co-infection with HIV. The increase in syphilis in men who have sex with men (MSM) has likely contributed to a decrease in relative racial/ethnic disparities in P&S syphilis because of the increased burden of syphilis in non-Hispanic White men. For example, in 2013, the black-to-white ratio for P&S syphilis was 5.3 among men and 15.0 among women.10
Our findings highlight two well-known potential drawbacks of using relative measures of racial/ethnic disparities in health to assess a program’s effectiveness in addressing these disparities. 5,24,25 First, relative measures of disparity can decrease due to increases in incidence rates among non-Hispanic whites. Consequently, an STD prevention program might judge itself successful in addressing disparities in a scenario in which the chosen disparity measure decreased solely due to increases in STDs among non-Hispanic whites. Second, whether or not racial and ethnic disparities increase from one year to the next can depend on one’s measure of disparity. Harper and colleagues, in their analysis of disparities in lung cancer over time, showed that one’s choice of disparity measure could influence one’s interpretation of changes in socioeconomic disparities in cancer and racial disparities in cancer.5 They noted that the use of a specific disparity measure to monitor trends in disparities requires careful thought and considerations of the characteristics of the given measure. 5 Further, the use of absolute measures of disparity as a complement to relative measures of disparity can help to provide a more complete assessment of disparities in health as well as changes in these disparities over time.7
Our analysis of trends in disparity measures is subject to limitations. First, there are limitations to the surveillance data we analyzed, such as under-reporting of cases, as described elsewhere.10 Differences in the degree of under-reporting across racial groups could bias our measurements of racial and ethnic disparities in STD rates. Second, we used bridged data so that we could examine reported STD rates for the same five racial/ethnic groups from 1981 to 2013. This approach could introduce bias over time due to factors such as the increased number of cases listed as “other” in the bridged data from 2007 to 2013. Cases reported as being more than one race (as is possible under the 1997 OMB standards) are mapped to “other” in the bridged data. If minority populations are more likely than non-Hispanic whites to be classified as more than one race, increases in the “other” classification over the time period of our analysis could lead to decreases in the assessed degree of racial/ethnic disparity in STDs, as we excluded cases listed as “other” in our main analyses. Third, the use of five racial/ethnic categories requires the combination of heterogeneous populations into single categories, such as “Asian Americans” and “Pacific Islanders.” Fourth, as noted above, we did not include all known measures of disparity.
Despite limitations, our analysis offers a new look at trends in disparities in STD rates over time in the US. All measures we assessed indicated a persistent and high degree of racial/ethnic disparity, consistent with the vast literature documenting these disparities.1–4 Our findings suggest that a wide range of disparity measures can be useful, individually or as a group, in providing a general overview of the degree of racial/ethnic disparities in STD rates. We found that the disparity measures we examined were generally consistent in assessing trends in disparity in reported STD rates, particularly when looking at changes over a longer time frame (5 years) rather than annual changes. However, our findings highlight potential drawbacks in the use of a single disparity measure to assess changes in disparities in STD rates from one year to the next or to measure a program’s performance in addressing racial/ethnic disparities in STD rates. In light of these findings, we recommend that programs (1) consider the use of more than one relative measure of disparity, and/or (2) ensure that the period of time over which trends are assessed is sufficient to capture the program’s impact on disparities. The choice of which disparity measure(s) to use will depend upon many factors, including subjective assessments by those who use these measures, such as whether or not to account for the population size of the racial/ethnic groups.
Supplementary Material
Summary.
Although the disparity measures we examined were generally consistent with one another, they sometimes differed in the magnitude and direction of change in disparity over time.
Acknowledgments
The authors thank Elizabeth Torrone and Hillard Weinstock for information about the bridged-race surveillance data and for suggesting the sensitivity analyses associated with this topic.
Footnotes
The authors have no conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26:250–61. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Dombrowski JC, Thomas JC, Kaufman JS. A study in contrasts: measures of racial disparity in rates of sexually transmitted disease. Sex Transm Dis. 2004;31:149–53. doi: 10.1097/01.olq.0000114656.57682.f4. [DOI] [PubMed] [Google Scholar]
- 3.St Louis ME, Farley TA, Aral SO. Untangling the persistence of syphilis in the South. Sex Transm Dis. 1996;23:1–4. doi: 10.1097/00007435-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chesson HW, Kent CK, Owusu-Edusei K, Jr, Leichliter JS, Aral SO. Disparities in sexually transmitted disease rates across the “eight Americas”. Sex Transm Dis. 2012;39:458–64. doi: 10.1097/OLQ.0b013e318248e3eb. [DOI] [PubMed] [Google Scholar]
- 5.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman ME. An overview of methods for monitoring social disparities in cancer with an example using trends in lung cancer incidence by area-socioeconomic position and race-ethnicity, 1992–2004. Am J Epidemiol. 2008;167:889–99. doi: 10.1093/aje/kwn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearcy JN, Keppel KG. A summary measure of health disparity. Public Health Rep. 2002;117:273–80. doi: 10.1016/S0033-3549(04)50161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regidor E. Measures of health inequalities: part 2. J Epidemiol Community Health. 2004;58:900–3. doi: 10.1136/jech.2004.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regidor E. Measures of health inequalities: part 1. J Epidemiol Community Health. 2004;58:858–61. doi: 10.1136/jech.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoover K, Bohm M, Keppel K. Measuring disparities in the incidence of sexually transmitted diseases. Sex Transm Dis. 2008;35:S40–4. doi: 10.1097/OLQ.0b013e3181886750. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2013. Atlanta: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 11.Aral SO. The social context of syphilis persistence in the southeastern United States. Sex Transm Dis. 1996;23:9–15. doi: 10.1097/00007435-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Colander DC. Economics. 5th. New York: McGraw-Hill; 2004. [Google Scholar]
- 13.Kerani RP, Handcock MS, Handsfield HH, Holmes KK. Comparative geographic concentrations of 4 sexually transmitted infections. Am J Public Health. 2005;95:324–30. doi: 10.2105/AJPH.2003.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott LJ, Blanchard JF, Beaudoin CM, et al. Geographical variations in the epidemiology of bacterial sexually transmitted infections in Manitoba, Canada. Sex Transm Infect. 2002;78(Suppl 1):i139–i44. doi: 10.1136/sti.78.suppl_1.i139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesson HW, Sternberg M, Leichliter JS, Aral SO. The distribution of chlamydia, gonorrhoea and syphilis cases across states and counties in the USA, 2007. Sex Transm Infect 2010. 86(Suppl 3):iii52–7. doi: 10.1136/sti.2009.040873. [DOI] [PubMed] [Google Scholar]
- 16.Chesson HW, Sternberg M, Leichliter JS, Aral SO. Changes in the state-level distribution of primary and secondary syphilis in the USA, 1985-2007. Sex Transm Infect 2010. 86(Suppl 3):iii58–62. doi: 10.1136/sti.2009.040865. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard JF, Halli S, Ramesh BM, et al. Variability in the sexual structure in a rural Indian setting: implications for HIV prevention strategies. Sex Transm Infect. 2007;83(Suppl 1):i30–6. doi: 10.1136/sti.2006.023572. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard JF, Khan A, Bokhari A. Variations in the population size, distribution and client volume among female sex workers in seven cities of Pakistan. Sex Transm Infect. 2008;84(Suppl 2):ii24–ii7. doi: 10.1136/sti.2008.033167. [DOI] [PubMed] [Google Scholar]
- 19.Leichliter JS, Chesson HW, Sternberg M, Aral SO. The concentration of sexual behaviours in the USA: a closer examination of subpopulations. Sex Transm Infect. 2010;86(Suppl 3):iii45–51. doi: 10.1136/sti.2010.042283. [DOI] [PubMed] [Google Scholar]
- 20.Keppel KG, Pearcy JN, Wagener DK. Healthy People 2000 statistical notes. National Center for Health Statistics; 2002. Trends in racial and ethnic-specific rates for the health status indicators: United States, 1990-98; pp. 1–16. [PubMed] [Google Scholar]
- 21.Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol. 1988;128:1185–97. doi: 10.1093/oxfordjournals.aje.a115073. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki E, Yamamoto E, Tsuda T. On the relations between excess fraction, attributable fraction, and etiologic fraction. Am J Epidemiol. 2012;175:567–75. doi: 10.1093/aje/kwr333. [DOI] [PubMed] [Google Scholar]
- 23.Anand S, Diderichsen F, Evans T, Shkolnikov VM, Wirth M. Measuring disparities in health: methods and indicators. In: Evans TWM, Diderichsen F, Bhuiya A, Wirth M, editors. Challenging inequities in health: from ethics to action. New York: Oxford University Press; 2001. pp. 49–67. [Google Scholar]
- 24.Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Soc Sci Med. 1997;44:757–71. doi: 10.1016/s0277-9536(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 25.Wagstaff A, Paci P, van Doorslaer E. On the measurement of inequalities in health. Soc Sci Med. 1991;33:545–57. doi: 10.1016/0277-9536(91)90212-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


