Table 2. Primary and secondary endpoints.
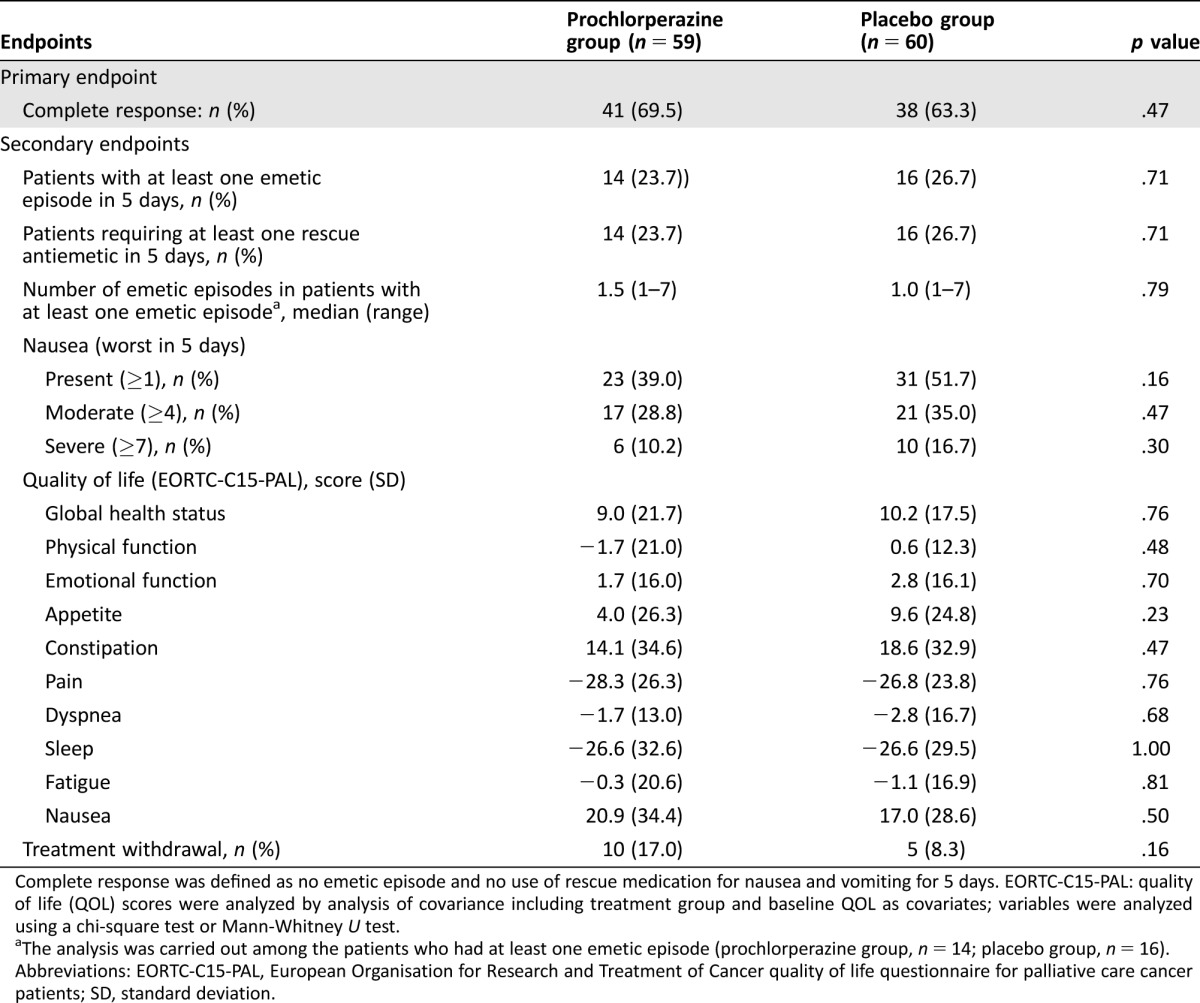
Complete response was defined as no emetic episode and no use of rescue medication for nausea and vomiting for 5 days. EORTC‐C15‐PAL: quality of life (QOL) scores were analyzed by analysis of covariance including treatment group and baseline QOL as covariates; variables were analyzed using a chi‐square test or Mann‐Whitney U test.
The analysis was carried out among the patients who had at least one emetic episode (prochlorperazine group, n = 14; placebo group, n = 16).
Abbreviations: EORTC‐C15‐PAL, European Organisation for Research and Treatment of Cancer quality of life questionnaire for palliative care cancer patients; SD, standard deviation.
