Figure 2.
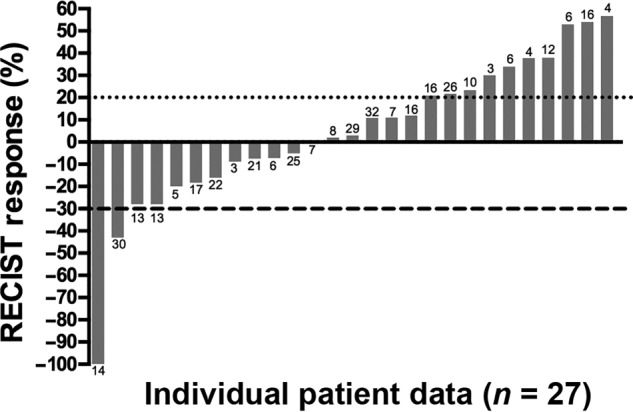
Antineoplastic effect of pazopanib in desmoplastic small round cell tumor per RECIST criteria. The percentage of reduction in tumor burden from baseline imaging in 26 patients to time of best response during the study period. Column labels indicate overall survival rounded to the nearest whole month for each patient from start of treatment. Cutoffs for partial response (>30% reduction in tumor volume) and progressive disease (>20% growth in tumor volume) as defined by RECIST 1.1 are noted with dotted (•) and dashed (‐) lines, respectively. Two patients with progressive disease are excluded from this graph: one patient who had immeasurable disease progression and one patient who died prior to follow‐up imaging.
Abbreviation: RECIST, Response Evaluation Criteria In Solid Tumors.
