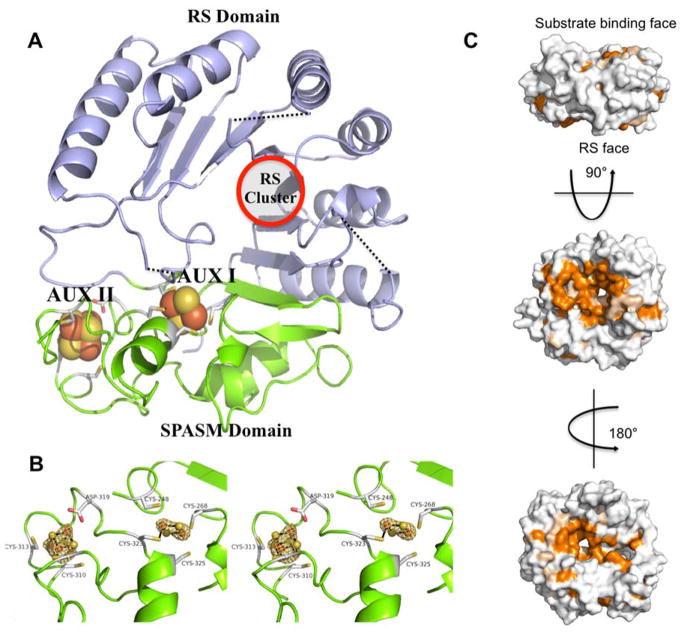
Figure 2.
Structure of MePqqE. (A) The Radical SAM (RS) domain (lavender) contains the RS (β/α)6 partial TIM barrel. The RS cluster and canonical motif are missing from the structure, but the location of the cluster has been approximated using a superposition of the AnSMEcpe structure as a model. The SPASM domain (green) harbors two auxiliary clusters. (B) Stereo view of the MePqqE SPASM domain, which contains a [2Fe-2S] cluster ligated by four cysteines and one [4Fe-4S] cluster ligated by three cysteines and one conserved aspartate. The Fe-edge (9.686 keV) anomalous difference electron density map (gold mesh) is contoured at 4.0 RMSD. (C) Surface representation of sequence conservation as calculated by the ConSurf server [http://conseq.bioinfo.tau.ac.il/] between MePqqE and 1061 members of the PqqE family (SFLD, http://sfld.rbvi.ucsf.edu/django/) using pairwise alignment. Strictly conserved residues are shown in orange, neutral substitutions are shown in tan, and variable regions are shown in white. The view is rotated 90° about the horizontal axis (middle) and 180° about the vertical axis (bottom).