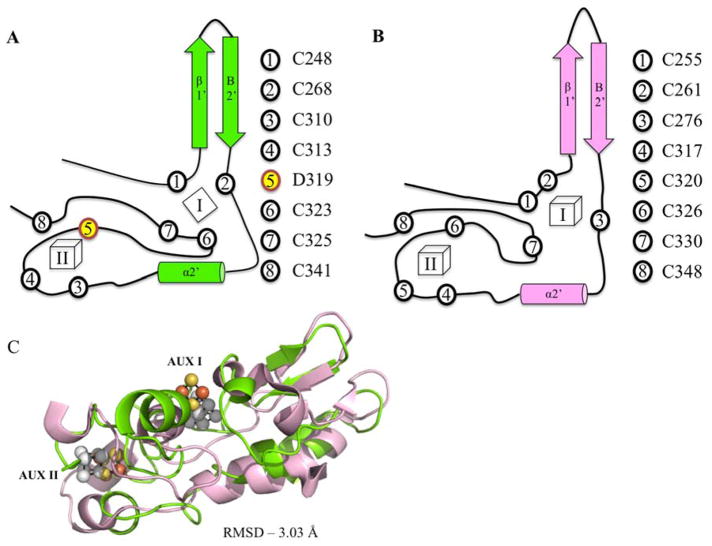
Figure 3.
Structural similarity of MePqqE and AnSMEcpe. Secondary structure and topology of MePqqE (A) and AnSMEcpe (B) show the ligation of auxiliary clusters. AUX I and AUX II are labeled as I and II. Ligating residues are represented by circles and corresponding residue numbers are shown to the right of each topological rendering. Asp 319 is shown by number 5, yellow highlighting. Panel (C) Shows the structural alignment of the SPASM domains of MePqqE in green (Fe is orange and S is yellow) and AnSMEcpe in pink (Fe and S are grey; RMSD 3.03 Å over 78 C-alphas, sequence identity 19.23%).